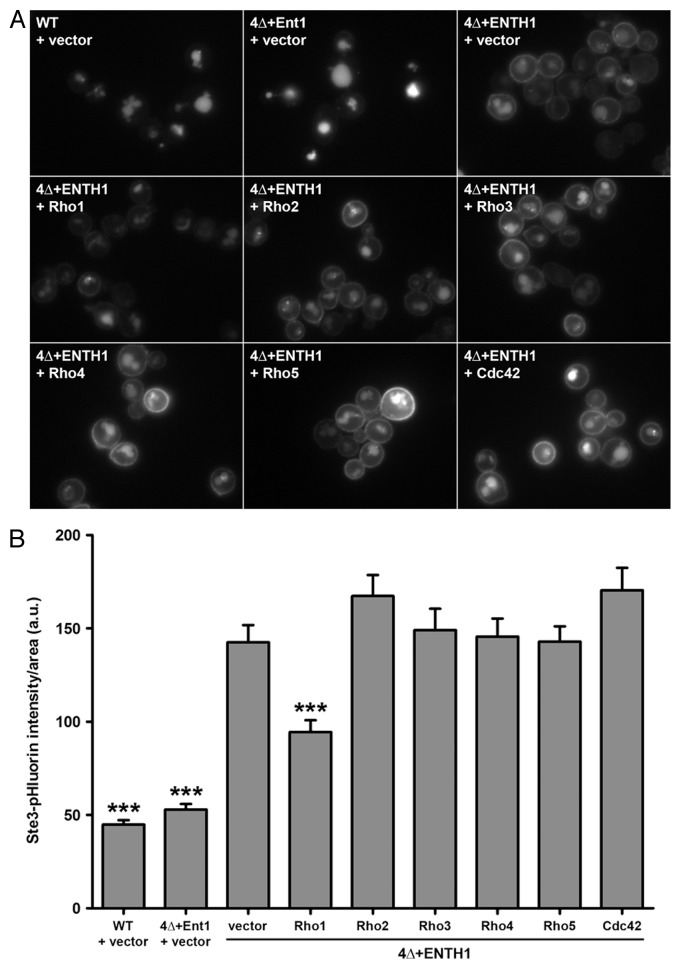
Figure 1. Specificity for Rho1 as a high-copy suppressor of endocytic defects in 4Δ+ENTH1 cells. (A) Wild-type (WT), 4Δ+Ent1 and 4Δ+ENTH1 cells expressing Ste3-GFP were transformed with empty vector or high-copy plasmids containing RHO1, RHO2, RHO3, RHO4, RHO5, or CDC42 to overexpress individual Rho GTPases as indicated. Cells were imaged by fluorescence microscopy to visualize Ste3-GFP, the a-factor mating pheromone receptor that is constitutively internalized and targeted to the vacuole in WT cells, but is partially retained at the plasma membrane when endocytosis is disrupted. (B) WT, 4Δ+Ent1 and 4Δ+ENTH1 cells expressing Ste3-pHluorin were transformed as described in panel A, and Ste3-pHluorin fluorescence intensity was quantified, with intensity values corrected for cell size. We recently developed a method for quantification of endocytosis in yeast cells using super-ecliptic pHluorin as a pH-sensitive GFP variant that becomes quenched in the acidic vacuole, allowing measurement of only fluorescently-tagged cargos that are outside of the vacuole.18 While cells with intact CME (WT and 4Δ+Ent1) are dim because Ste3-pHluorin is mainly localized in the vacuole, cells with defective CME (4Δ+ENTH1) are comparatively bright because a significant population of Ste3 is retained at the cell surface. Values are presented as mean ± SEM from a minimum of 30 cells per condition, and statistical significance was assessed by one-way ANOVA with Neuman-Keuls post hoc analysis (*** p < 0.001 compared with 4Δ+ENTH1 + vector). All strains used in this analysis were described previously.15

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.