Figure 2. GreA facilitates protein reactivation from unfolded state.
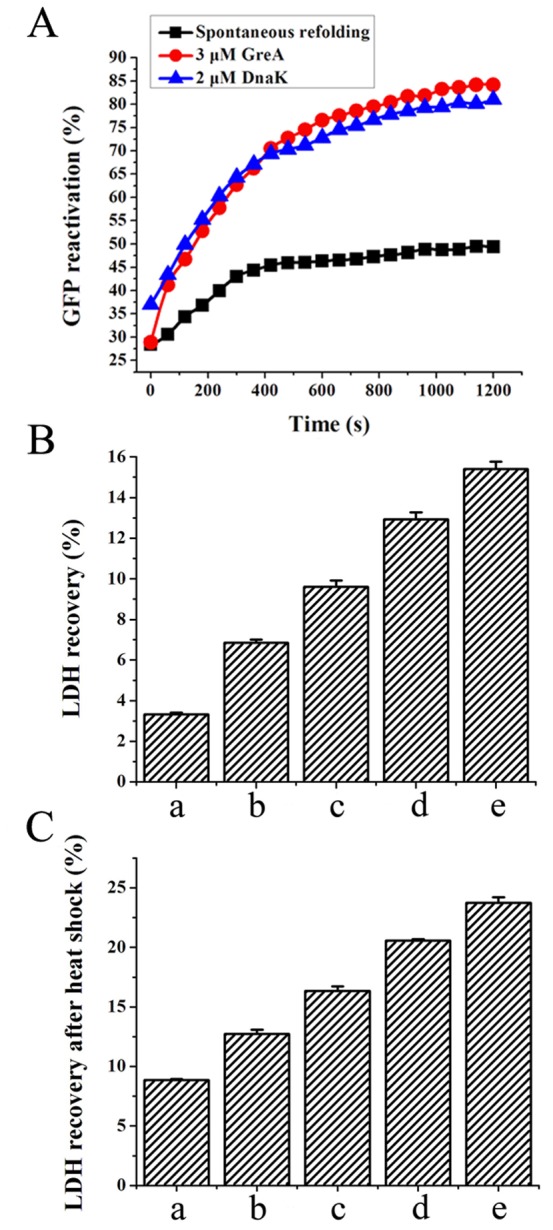
(A) GreA facilitates GFP refolding. GFP (100 µM) was denatured in 0.12 M HCl for 60 min and then diluted 100-fold. Spontaneous refolding or in the presence of 3 µM GreA or 2 µM DnaK was monitored using a Fluostar Optima microplate reader. (B) GreA promotes LDH refolding after GnHCl denaturation. LDH (15 µM) denatured by 6 M GnHCl was diluted 100-fold to start spontaneous refolding or GreA-facilitated refolding. (a) Control (b) 0.3 µM GreA (c) 0.6 µM GreA (d) 1.2 µM GreA (e) 1.2 µM DnaK. (C) GreA promotes LDH refolding after heat denaturation. 0.2 µM LDH was incubated at 50°C for 80 min. After cooling down, 0.2 µM, 0.4 µM, 0.8 µM GreA or 0.5 µM DnaK was added to start refolding and the final concentration of LDH was adjusted to 0.1 µM. The enzymatic activity was detected after recovery for 30 min. (a) Control (b) 0.2 µM GreA (c) 0.4 µM GreA (d) 0.8 µM GreA (e) 0.5 µM DnaK.
