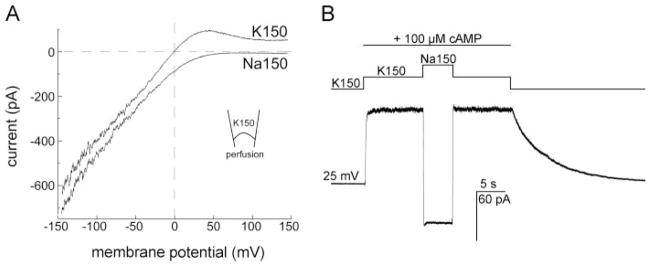
FIGURE 5. The cAMP-activated MmaK current rectifies inwardly and is K+-specific.
A, ensemble currents during a 200-ms voltage ramp from −150 to +150 mV show that the MmaK current rectifies inwardly. The ~370 channels in the excised patch were activated by 100 μM cAMP at 0 mV for 3 s before the voltage ramp was applied. Leak currents of the entire ramp in the absence of cAMP activation were subtracted. In symmetric 150 mM KCl (K150, upper trace, perfusate contains cAMP), the outward current is reduced to near zero at positive voltages above ~100 mV, indicative of inward rectification. Between 0 and +75 mV, however, outward currents are observed, indicative of open channels. (Here outward current flows from the bath, the cytoplasmic side, into the pipette.) As the voltage increases, rectification reduces the ensemble conductance while the outward electromotive force increases. This competition results in the prominent hump. Replacing the perfusate with cAMP-containing 150 mM NaCl (Na150, lower trace) eliminates this hump, indicating that the MmaK channel still rectifies but there is no outward driving force for cation when K+ is absent. The downward displacement of the current trace in Na150 at negative voltages is also consistent with the increase in the chemical driving force of K+ through a K+-selective filter. Sampled at 10 kHz and filtered at 4 kHz. B, switching the cAMP-containing perfusate from K150 to Na150 reverses the macroscopic current at +25 mV, further illustrating that MmaK does not allow Na+ permeation. Sampled at 1 kHz and filtered at 0.5 kHz.