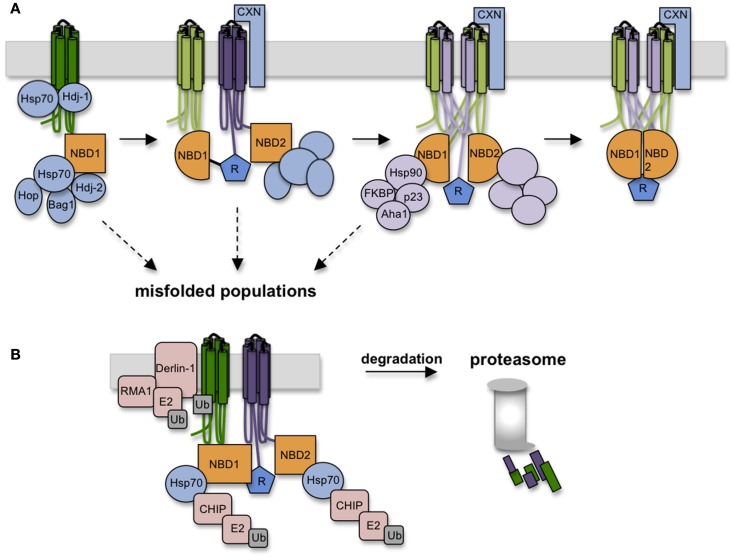
Figure 5.
Chaperones assist CFTR folding and target misfolded CFTR for degradation. (A) As CFTR is synthesized, numerous chaperones and co-chaperones (some depicted here) decorate the nascent polypeptide on both lumenal and cytosolic sides of the ER membrane. Hsp70 and co-chaperones interact with NBD1, followed by calnexin association with TMD2. Hsp70-Hop interactions recruits Hsp90 complexes which likely aid domain assembly in conjunction with calnexin. (B) Failure to achieve productive folding at any step in the folding pathway is detected by persistent binding of Hsp70, which serves to recruit E3 ligases (i.e., RMA1 and CHIP) that ubiquitinate CFTR and target it to the 26S proteasome.