Abstract
Introduction
Myrtol standardized is established in the treatment of acute and chronic bronchitis and sinusitis. It increases mucociliar clearance and has muco-secretolytic effects. Additional anti-inflammatory and antioxidative properties have been confirmed for Myrtol standardized, eucalyptus oil, and orange oil in several in vitro studies.
Objective
The aim of this study was to prove the ability of essential oils to reduce cytokines release and reactive oxygen species (ROS) production derived from ex vivo cultured alveolar macrophages.
Material and methods
Alveolar macrophages from patients with chronic obstructive pulmonary disease (COPD, n = 26, GOLD III-IV) were pre-cultured with essential oils (10-3-10-8%) for 1 h and then stimulated with LPS (1 μg/ml). After 4 h and 20 h respectively a) cellular reactive oxygen species (ROS) using 2',7'-dichlorofluorescein (DCF), and b) TNF-α, IL-8, and GM-CSF secretion were quantified.
Results
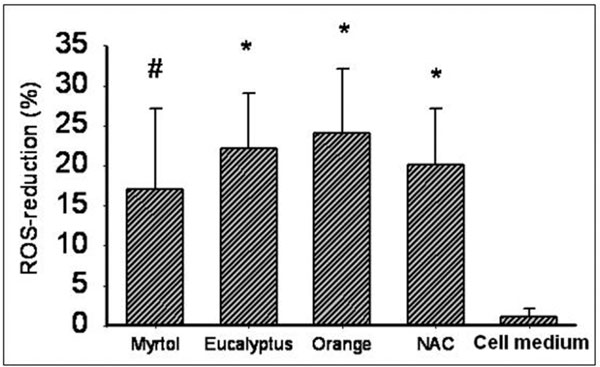
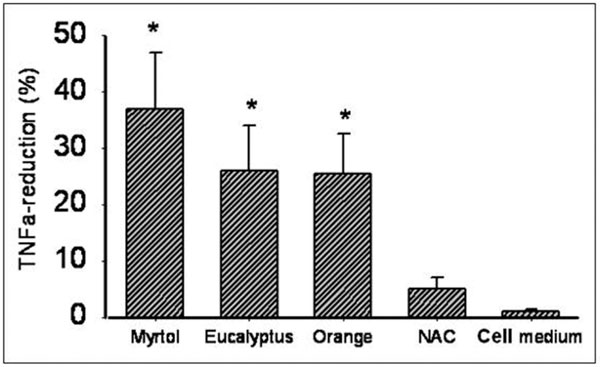
In comparison with negative controls, pre-cultured Myrtol, eucalyptus oil and orange oil (10-4%) reduced in the LPS-activated alveolar macrophages ROS release significantly after 1+20 h as follows: Myrtol - 17.7% (P = 0.05), eucalyptus oil -21.8% (P < 0.01) and orange oil -23.6% (P < 0.01). Anti-oxidative efficacy was comparable to NAC (1 mmol/l). Essential oils also induced a TNF-α reduction: Myrtol (-37.3%, P < 0.001), eucalyptus oil (-26.8%, P < 0.01) and orange oil (-26.6%, P < 0.01). TNF-α reduction at 1+4 h and 1+20 h did not vary (Myrtol: -31.9% and -37.3% respectively, P = 0.372) indicating that this effect occurs early and cannot be further stimulated. Myrtol reduced the release of GMCSF by -35.7% and that of IL-8 only inconsiderably.
Conclusions
All essential oils tested have effective antioxidative properties in ex vivo cultured and LPS-stimulated alveolar macrophages. Additionally, Myrtol inhibited TNF-α and GM-CSF release best indicating additional potent anti-inflammator y activity.
Keywords: essential oils, myrtol, eucalyptus oil and orange oil, COPD, alveolar macrophages, cytokines, reactive oxygen species
Introduction
Chronic bronchitis is a subset of a chronic obstructive pulmonary disease (COPD) [1]. It is clinically diagnosed by the presence of cough as well as productive sputum for at least three months during two or more consecutive years [2]. Chronic exposure to cigarette smoke damages the airway epithelium, leading to squamous metaplasia. Epithelial layer thickness, promoted by epithelial cell hyperplasia, hypertrophy and mucous metaplasia increases incrementally as disease severity worsens. Inflammatory cells are routinely observed in the tissue and the airways of COPD patients, whereas neutrophils are the most abundant cellular population [3]. Airway mucus hypersecretion is a key pathophysiological feature in most COPD patients. Consequently, many drugs have been developed, either to inhibit mucus hypersecretion or to reduce the viscosity to ease mucus elimination by cough. Some have been reported to have anti-inflammatory properties such as N-acetylcysteine (NAC) and its derivatives or ambroxol [4-6]. Clinical efficacy of mucolytic drugs was discussed somewhat controversial. Regardless of numerous positive results from the 1980s and promising metaanalysis [7,8], one newer placebo controlled and randomized trial with NAC is fairly disappointing [9]. As a consequence mucolytic drugs are not generally recommended for COPD treatment [10].
Phytomedicines and herbal remedies have a long history in the treatment of COPD and of patients suffering from bronchitis. Herbal medicine is very popular, but only a few studies analysed the underlying mechanism of their efficacy [11]. Among those, Myrtol (CAS-No. 8002-55-9), a muco-secretolytic phytomedicine containing the monoterpenes α-pinene, dlimonene and 1,8-cineole as marker substances, provides proven efficacy in the treatment of acute and chronic bronchitis [7,12-14]. The aim of the present study was the investigation of Myrtol and some other essential oils in having anti-inflammatory properties on human alveolar macrophages.
Materials and methods
Study Design
The study was approved by the Saxonian Ethics Committee in Dresden, Germany (EK-BR-27/05-2). Myrtol, eucalyptus oil and orange oil were tested in an open, single-center and case-controlled study to reduce certain pro-inflammatory parameters in isolated alveolar macrophages. The cells were derived from COPD patients (n = 26) by bronchoscopy and bronchoalveolar lavage (BAL), using a standard method as reported earlier [15,16]. The patients were all recruited from the Robert-Koch-Hospital, an academic teaching hospital and integral part of the St. George Medical Center, in Leipzig, Germany, specialized in pulmonary medicine including lung cancer. COPD was defined according to the Global Initiative for Lung Disease [10]. Only patients with GOLD stage III-IV were eligible to ensure high cellular activity due to persistent inflammation in the airways. All patients gave their informed consent prior to inclusion. They were all on regular treatment with inhaled long-acting β2-agonists, tiotropium bromide, and theophylline and inhaled corticosteroids. None received systemic corticosteroids or mucolytics. Baseline characteristics of the population studied and of the BAL content are shown in Table 1.
Table 1.
Baseline data and cellular broncho-alveolar lavage (BAL) content from the study population.
| Mean | Range | Median | Median SD | |
|---|---|---|---|---|
| Patients (n) | 26 | - | - | - |
| Male/female (n) | 17/9 | - | - | - |
| Age (years) | 63.9 | 41-79 | 62.4 | 8.5 |
| BMI (kg/m2) | 26.5 | 19-34 | 24.5 | 2.5 |
| Smoker-current (%) | 21.9 | - | - | - |
| Former-smoker (%) | 41.7 | - | - | - |
| Smoker (pack-years) | 19.2 | 10-35 | 17.5 | 7.9 |
| Former smoker (pack-years) | 21.0 | 10-35 | 20.0 | 8.8 |
| Lung function | ||||
| FEV1 (l) | 2.2 | 0.6-4.1 | 2.1 | 1.1 |
| FEV1 (%predicted) | 73.5 | 29.7-124.6 | 70.5 | 26.8 |
| VC max (l) | 2.8 | 0.83-5.1 | 2.8 | 1.2 |
| VC max (%predicted) | 73.7 | 43.20-126.0 | 73.2 | 22.8 |
| FEV1/VC max | 78.6 | 45.39-98.6 | 81.4 | 12.0 |
| RV (l) | 2.4 | 1.08-5.4 | 2.1 | 1.0 |
| RV (%predicted) | 112.4 | 61.5-226.6 | 107.0 | 40.5 |
| BAL | ||||
| Total cells (106/100 ml) | 29.7 | 10-85 | 28.8 | 16.9 |
| Macrophages (%) | 61.2 | 28-90 | 63.5 | 19.2 |
| Neutrophils (%) | 12.4 | 1-50 | 6.0 | 12.7 |
| Eosinophils (%) | 2.0 | 0-14 | 1.0 | 3.2 |
| Lymphocytes (%) | 17.2 | 4-62 | 15.0 | 11.8 |
Experimental Protocol
The purity of isolated alveolar macrophages was verified using an inverse fluorescence microscope (Nikon Eclipse TS100, type 120) after pre-incubation in which viable cells adhere to the bottom of the tissue culture plate. Alveolar macrophages viability, before and after the incubation with essential oils, was determined by trypan blue exclusion (< 95%). The highest concentration of essential oils used had no adverse effects on cell viability. alveolar macrophages were pre-incubated in 96-well tissue culture plates (105 per well filled with 1 ml RPMI, fetal calf serum [FCS, 10%], 1%w/v penicillin/streptomycin/amphotericin/1% L-glutamine) for up to 20 h. Myrtol, orange oil, eucalyptus oil (10-3-10-8%, all a generous gift from G. Pohl-Boskamp GmbH & Co. KG, Hohenlockstedt/Germany) diluted in RPMI were than added for 1 h. After washing, the cells were further incubated in RPMI/1%FCS/penicillin/streptomycin/amphotericin/L-glutamin and LPS (1 μg/ml) for the next 4 h (= 1+4 h) and 20 h (= 1+20 h) respectively. Conditioned media were than used for further analysis (below). The concentrations of essential oils and LPS were determined in pre-experiments. They also were adapted to known concentrations in blood [14].
Antioxidative Capacity
Antioxidative properties of essential oils were tested as a function of inhibited release of cellular reactive oxygen species (ROS). ROS were quantified using the fluorescence marker 2',7'-dichlorofluorescein (DCF, Merck, Darmstadt, Germany). The intensity of fluorescence measured corresponds to ROS concentration as reported earlier [17]. ROS generation was estimated as the maximal DCF mediated fluorescence of 2 minutes at 37°C with the excitation and emission wavelenghts at 488 and 520 nm respectively (fluorophotometer FLUOstar Optima, BMG Labtech, Offenburg, Germany). In pre-experiments serial dilutions of reagent DCF were monitored on the fluorometer to obtain a standard curve of fluorescence per nanomole DCF. ROS produced by alveolar macrophages were expressed as picomoles DCF per 105 cells and - in terms of antioxidative capacity of the oils used - presented in % reduction. Because of its already proven antioxidative properties NAC (1 mmol/l; Sigma Chemicals) was used as a positive control [4]. The negative control consisted of cell medium only.
Mediator Release
Mediator release from cultured alveolar macrophages was quantified using enzyme linked immunosorbent assays according to the manufacturer's protocols (ELISA). We measured TNF-α (Immuno Tools, Friesoythe, Germany), IL-8 and GM-CSF (IBL, Hamburg, Germany).
Statistical Methods
The effects of potential interacting variables (essential oils with different concentrations and incubation times) on outcomes were assessed using two-way ANOVA. Univariate comparisons were made using the Student's t-test. Log transformation was used for stabilization of variance, as required. SigmaStat 3.1 software (SPSS cooperation) was used for all statistical calculations. Error bars in graphs represent the standard deviation of the mean.
Results
Effect of ROS Reduction
In comparison with the negative control, pre-cultured Myrtol, eucalyptus oil and orange oil (10-4%) reduced ROS in LPS-activated alveolar macrophages significantly: Myrtol (P < 0.05), eucalyptus oil and orange oil (P < 0.01; Figure 1).
Figure 1.
Reactive oxygen species (ROS) reduction in LPS (1 μg/ml)-stimulated alveolar macrophages from COPD patients with various essential oils (0.0001% dilution) at 1+20 h incubation time (see text for details). Data presented as means ± SD, NAC - N-acetylcysteine (1 mmol/l), orange - orange oil, eucalyptus - eucalyptus oil. #P < 0.05, *P < 0.01 compared with cell medium/LPS alone, non-significant differences among essential oils and NAC.
Effect of TNF-α Reduction
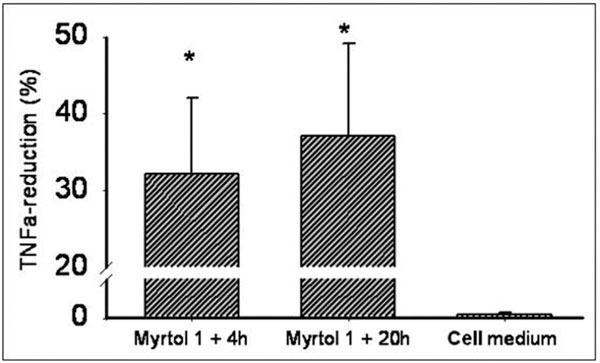
Myrtol reduced cellular TNF-α release in a 0.0001% dilution (incubation time 1+20 h) by 37.3% (P < 0.01; Figure 2). Again, there was no statistical difference between Myrtol, eucalyptus and orange oil. Maximal TNF-α reduction by Myrtol was already reached after 1+4 h: 31.9% reduction (P < 0.01, compared with medium alone; P = 0.37 compared with 1+20 h, Figure 3).
Figure 2.
Reduction of TNFα release from LPS (1 μg/ml-stimulated alveolar macrophages from COPD patients at 1+20 h incubation time (see text for details). TNFα - tumor necrosis factor alpha, NAC - N-acetylcysteine (1 mmol/l), orange - orange oil, eucalyptus - eucalyptus oil. *P < 0.01 compared with cell medium/LPS alone.
Figure 3.
Reduction of TNFα release from LPS (1 μg/ml)-stimulated alveolar macrophages derived from COPD patients. Incubation time: 1 h pre-incubation with myrtol 0.0001% solution, followed by a washing step and 4 h incubation (in RPMI/fetal calf serum/LPS). Differences non-significant between myrtol incubation times, but P < 0.01 (*) compared with cell medium alone. TNFα - tumor necrosis factor alpha.
Effect of IL-8 and GM-CSF Reduction by Myrtol
Due to low cell number and limited amount of cell medium per well, we focused on Myrtol to be tested for IL-8 and GM-CSF reduction. Myrtol (0.0001%, 1+20 h) reduced cellular IL-8 release by 12.3% and GM-CSF-secretion by 35.8%.
Discussion
In the present study, we confirmed anti-inflammatory properties of essential oils. Our results demonstrate that particularly Myrtol at known therapeutic blood concentrations was a surprisingly strong inhibitor of TNF-α and GM-CSF, but was also able to reduce the concentration of the chemotactic mediator IL-8 and ROS.
For 1,8-cineole, one of the main monoterpenes and key biological marker substance of Myrtol, a reduction of cellular TNF-α release in PMA (12-myristate 13-acetate, 10 nM)-activated lymphocytes and in LPS (10 μg/ml)-treated monocytes has been shown earlier [18]. Due to differences in the experimental setup and mediators measured, different cell population and cell source, and varying information on the dosage of the essential oils tested, it seems impractical to compare their data with ours. Nonetheless, both studies clearly indicate anti-inflammatory properties of terpenoids, despite the differences.
Graßmann et al. [14] reported a Myrtol and eucalyptus oil-driven reductions of hypochlorous acid (HOCl) in a system based on the degranulation of activated neutrophilic granulocytes isolated from the whole blood. Myrtol and eucalyptus oil also inhibited peroxynitrite (ONOO-) formation indicating that both oils interact with aggressive oxygen radicals from the OH•-type [14].
In an arachidonic model, oral administration of Myrtol results in a dose-dependent reduction in the leukotriene concentration (LTC4/D4/E4) [19]. A marked reduction in the rise in PGE2 induced by an inflammatory stimulus (TPA application) further confirms the anti-inflammatory properties of Myrtol in an isolated perfused bovine udder model. In supplementary investigations on basophilic leukaemia cells in rats, Myrtol inhibited 5-lipoxygenase, another key enzyme in the inflammatory cascade [19]. Our data are therefore in good accordance with earlier reports, although we used an alveolar macrophages based system measuring fluorescence as a non-selective method to compute ROS, rather than a cell free system using gas chromatography as Graßmann et al. did [14]. Antioxidative properties of Myrtol were comparable with those of orange oil, eucalyptus oil, but also with NAC which we used as a positive control. NAC is well known for its direct (through oxidation of its molecular SH-group), but also for its indirect (precursor function of cellular glutathione formation) antioxidative potential [20-24]. The exact mechanism how essential oils exert this function on inflammatory cells is still unknown.
Our experimental design did not allow direct inhibition of oxygen radicals, e.g., via oxidation as known from NAC or glutathione. So, we assume that Myrtol, eucalyptus oil, and orange oil interfere with pro-inflammatory cell metabolism, eventually leading to a drop in cellular ROS and cytokine release. Because this effect is shown in alveolar macrophages from COPD patients, who are well known to suffer from high ROS burden and whose airways are further characterized by elevated cytokine levels [25-27], Myrtol may function as a logical therapeutic choice. However, it still needs to be investigated to what extent essential oils given to patients reveal anti-inflammatory function in the airways, and if so, whether this effect directly correlates with clinical measurable benefits for the patients, beside its proven mucolytic efficacy.
Earlier studies indicate that Myrtol standardized protects against COPD exacerbations [7,12,13]. The authors stated that mucolytic ability may have been responsible for this effect. Considering our results and earlier reports [14,19], it can be assumed that anti-inflammatory properties of Myrtol may have contributed to the reduction of exacerbation rates in COPD as reported from earlier trials [13].
The present study has various disadvantages. Our single data point varied considerably as indicated by large standard deviation, being predominately related to the cell source. The quality of lavaged alveolar macrophages is not always uniform, due to various reasons related to the individual patients, their smoking status, medication taken, disease stage, co-morbidity, and the patient's performance during flexible bronchoscopy. To cope with these problems, we measured our samples in triplicates, causing a shortage of available cells. Due to a limited number of alveolar macrophages, we were unable to perform all assays simultaneously with the three essential oils. For that reason, we tested only Myrtol for IL-8 and GM-CSF reduction, but not eucalyptus oil and orange oil. All in all, we were able to confirm previous reports that essential oils and, particularly, Myrtol have anti-inflammatory properties.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
Supported by a research grant from G. Pohl-Boskamp GmbH & Co. KG, Hohenlockstedt, Germany.
References
- Rabe KF, Hurd SS, Anzueto A, Barnes PJ, Buist SA, Calverley PMA, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, Weel van C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD Executive Summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report of an expert committee: Definition and diagnosis of pulmonary disease with special reference to chronic bronchitis and emphysema. WHO Techn Rep Ser. 1961;213:14–9. [Google Scholar]
- Patel IS, Roberts NJ, Lloye-Owen SJ, Sapsford RJ, Wedzicha JA. Airway epithelial inflammatory responses and clinical paramters in COPD. Eur Respir J. 2003;22:94–9. doi: 10.1183/09031936.03.00093703. [DOI] [PubMed] [Google Scholar]
- Gillissen A, Bartling A, Schoen S, Schultze-Werninghaus G. Antioxidant function of ambroxol in mononuclear and polymorphonuclear cells in vitro. Lung. 1997;175:235–42. doi: 10.1007/PL00007570. [DOI] [PubMed] [Google Scholar]
- Nowak D, Antczak A, Krol M, Bialasiewicz P, Pietras T. Antioxidant properties of ambroxol. Free Radic Biol Med. 1994;16:517–22. doi: 10.1016/0891-5849(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Gillissen A, Nowak D. Characterization of N-acetylcyteine and ambroxol in antioxidant therapy. Resp Med. 1998;92:609–23. doi: 10.1016/S0954-6111(98)90506-6. [DOI] [PubMed] [Google Scholar]
- Poole PJ, Black PN. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic review. BMJ. 2001;322:1–6. doi: 10.1136/bmj.322.7277.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AB, Pavia D, Agnew JE, Lopez-Vidriero MT, Lauque D, Clarke SW. Effect of oral N-acetylcysteine on mucus clearance. Br J Dis Chest. 1985;79:262–6. [PubMed] [Google Scholar]
- Decramer M, Rutten-von-Molken MPMH, Dekhuijzen PNP, Schayck van CP, Olivieri D, Del Donno M, De Backer W, Lankhorst I, Ardia A. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCHUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–60. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- GOLD Executive Committee. Global initiative for chronic obstructive lung disease. 2007. http://www.goldcopd.com
- Guo X, Pittler MH, Ernst E. Herbal medicines for the treatment of COPD: a systematic review. Eur Respir J. 2006;28:330–8. doi: 10.1183/09031936.06.00119905. [DOI] [PubMed] [Google Scholar]
- Matthys H, de Mey C, Carls C, Rys A, Geib A, Wittig T. Efficacy and tolerability of myrtol standardized in acute bronchitis. A multi-centre, randomised, double-blind, placebo-controlled parallel group clinical trial vs. cefuroxime and ambroxol. Arzneim-Forsch/Drug Res. 2000;50:700–11. doi: 10.1055/s-0031-1300276. [DOI] [PubMed] [Google Scholar]
- Meister R, Wittig T, Beuscher N, de Mey C. Efficacy and tolerability of myrtol standardized in long-term treatment of chronic bronchitis. A double-blind, placebo-controlled study. Arzneim-Forsch/Drug Res. 1999;1:351–8. doi: 10.1055/s-0031-1300426. [DOI] [PubMed] [Google Scholar]
- Graßmann J, Hippeli S, Dornisch K, Rohnert U, Beuscher N, Elstner EF. Antioxidant properties of essential oils. Possible explanations for their anti-inflammatory effects. Arzneim-Forsch/Drug Res. 2000;50:135–9. [PubMed] [Google Scholar]
- Report of the European Society of pneumology task group on BAL. Technical recommendations and guidelines for bronchalveolar lavage (BAL) Eur Respir J. 1989;2:561–85. [PubMed] [Google Scholar]
- Haslam PL, Baughman RP. Report of ERS Task Force: guideline for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14:245–8. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- Delacourt C, d'Ortho MP, Macquin-Mavier I, Pezet S, Housset B, Lafuma C, Harf A. Oxidant-antioxidant balance in alveolar macrophages form newborn rats. Eur Respir J. 1996;9:2517–24. doi: 10.1183/09031936.96.09122517. [DOI] [PubMed] [Google Scholar]
- Juergens UR, Engelen T, Stober M, Gillissen A, Vetter H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in human mononuclear phagocytes in vitro. Pulm Phamacol Therapeutics. 2004;17:281–7. doi: 10.1016/j.pupt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Beuscher N, Kietzmann M, Bien E, Champeroux P. Interference of Myrtol standardized with inflammatory and allergic mediators. Arzneim-Forsch/Drug Res. 1998;48:985–9. [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-X. [DOI] [PubMed] [Google Scholar]
- Gillissen A, Jaworska M, Orth M, Coffiner M, Maes P, App EM, Cantin AM, Schultze-Werninghaus G. Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitro. Resp Med. 1997;91:159–68. doi: 10.1016/S0954-6111(97)90052-4. [DOI] [PubMed] [Google Scholar]
- Jaworska M, Gillissen A, Schärling B, Wickenburg D, Schultze-Werninghaus G. N-Acetylcystein: ein funktioneller Sauerstoffrädikalfanger in vitro und ex vivo in Monozyten und neutrophilen Granulozyten bei Patienten mit einer COPD. Pneumologie. 1995;49:539–45. [PubMed] [Google Scholar]
- Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicology. Am J Med. 1991;3C:131S–9S. doi: 10.1016/0002-9343(91)90296-a. [DOI] [PubMed] [Google Scholar]
- Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med. 1991;91(Suppl 3C):3C-54S–3C-59S. doi: 10.1016/0002-9343(91)90284-5. [DOI] [PubMed] [Google Scholar]
- Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–56. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- Sarir H, Henricks PAJ, Houwelingen AH, Nijkamp FP, Folkerts G. Cells, mediators and Toll-like receptors in COPD. Eur J Pharmacol. 2008;585:346–53. doi: 10.1016/j.ejphar.2008.03.009. [DOI] [PubMed] [Google Scholar]
- O'Donnell RA, Peebles C, Ward JA, Daraker A, Angco G, Broberg P, Pierrou S, Lund L, Holgate ST, Davies DE, Delany DJ, Wilson SJ, Djukanovic R. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–42. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]