Abstract
Oral cancer is one of the most common types of cancer seen in India with buccal and alveolo buccal regions being the most frequent subsites. A retrospective analysis of buccal and alveolo buccal cancer patients undergoing neck dissection from 1995 to 2009 was performed to analyze the profile of neck dissections and patterns of nodal involvement in these patients. Total 310 neck dissections were done for buccal and alveolo-buccal cancer including 41 (13.2 %) RND, 231(74.5 %) MND and 38 (12.2 %) Supraomohyoid neck dissection (SOHND). Clinically palpable nodes were present in 75.9 % patients but only 117 (38 %) were pathologically node positive. 20 % had occult positive nodes in N0 group. Level I was most commonly involved with 35 % having positive nodes in more than one level. There were no patients with isolated involvement of level IV or V with only 3.9 % patients with involvement of level III. Current guidelines recommend neck dissection in all clinically node positive patients. However, our experience shows that neck is over treated in majority of patients and there is a need to optimize surgical management of neck in these patients.
Keywords: Oral cancer, Node assessment, Neck node dissection
Introduction
Oral squamous cell cancer (OSCC) is one of the most common cancers in India [1]. The disease differs from western world with buccal and alveolo-buccal subsites and advanced stage at presentation more common in India [2]. Locally advanced disease is indeed a surgical challenge, as it requires a blend of radical excision with functional and cosmetic preservation. The strategy for management of neck nodes in these patients is equally important to attain optimum loco-regional control. The surgical management of neck nodes has evolved during last few decades from radical neck dissection to selective node dissections (SND) and sentinel lymph node biopsy (SLNB) procedures. The aim of this study was to analyze the patterns of neck dissection trends and clinical and pathologic node involvement in patients presenting with buccal and alveolo-buccal cancer.
Materials and Methods
A retrospective review of prospective computerized oral cancer database at department of Surgical Oncology, Dr BRA-IRCH, All India Institute of Medical sciences (AIIMS), Delhi was performed for patients presenting between 1995 and 2009 with oral cancer involving buccal or alveolo-buccal sub sites. Patients with previously untreated, pathologically proven, OSCC arising in buccal or alveolo-buccal site were included in the study. Patients with cancers arising at other subsites with extension to buccal mucosa or alveolus were excluded from the study. Patients who did not undergo a neck dissection and upper alveolus tumors were also excluded. Details of clinical examination and CT scan (whenever done) were recorded. These were then taken as basis for classifying patients into node negative (cN0) or node positive (cN+). All patients with clinically positive neck nodes underwent a comprehensive neck dissection (CND) in either RND or MND. Patients with clinically negative neck with high risk factors had a SOHND. All patients with pathological proven N+ neck were given adjuvant post-operative Radiotherapy (PORT).
The records of these patients were analyzed in relation to the profile of neck dissections and pattern of nodal involvement. All patients were restaged as per 2002 American Joint Committee on Cancer (AJCC) staging system. The stage of presentation, neck dissection performed and pathologic features of the neck dissection specimen including total nodes, positive nodes and pathologic stage were documented.
Results
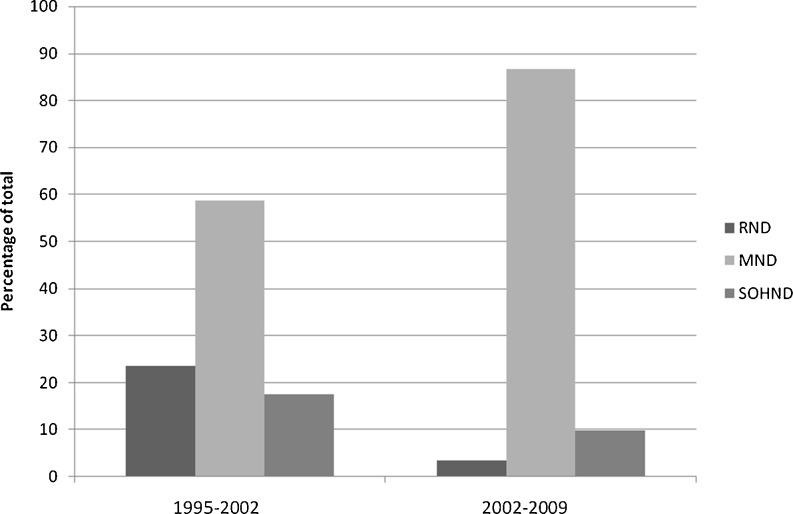
Seven hundred ninety four patients underwent surgery for OSCC between 1995 and 2009. Of these 794 patients, 313 patients (39.4 %) had primary buccal and alveolo-buccal cancer. Out of 313 patients, 303 patients underwent 310 neck dissections. Seventy four percent of patients presented in an advanced stage (stage III and IVA) of the disease. Majority (75.9 %) of the patients were cN+ with only 24.1 % being cN0. During the early part of the study, a higher percentage of patients underwent RND during the initial part of the study. This trend (Fig. 1) changed in the later years in tune with the changing guidelines of neck dissections. Table 1 provides the details of these procedures.
Fig. 1.
Time trends of Neck Dissection Procedure
Table 1.
Neck Dissection Types
| RND | MND | SOHND | |
|---|---|---|---|
| Patients (Percentage) | 41 (13.2 %) | 231 (74.5 %) | 38 (12.2 %) |
| Mean Nodal Yield (Range) | 12 (1-12) | 17 (1-55) | 10 (1-25) |
| Pathologically positive dissections | 53 % | 39 % | 14.7 % |
RND: radical Neck Dissection, MND: Modified radical neck dissection, SOHND: Supraomohyoid neck dissection
Among this entire cohort of buccal and alveolo-buccal cancers, only 113 patients (37.2 %) had positive lymph nodes on pathologic analysis of the neck dissection specimen (pN+). Of the 239 patients (79.5 %) in cN+ group 57.4 % did not have any pathologic positive lymph nodes (Table 3). The sensitivity of clinical examination for detection of neck nodes was 86.7 % but the specificity was a mere 30.5 %. Hence, even though only a minority of cN+ patients eventually had positive lymph nodes on pathologic examination, all were subject to a MND I procedure with its associated morbidities. Fifteen patients (20.5 %) in cN0 group had occult neck secondary. The rate of occult neck secondary was 17.6 % and 23 % in early and advanced T stages, respectively (Table 2).
Table 3.
Incidence of False Positive neck in N+, T stage wise distribution
| T stage (number of patients) | Percentage |
|---|---|
| T1 (07) | 71.4 |
| T2 (35) | 65.7 |
| T3 (27) | 85.2 |
| T4(161) | 50.3 |
Table 2.
Incidence of False Negative neck in N0 neck, T stage wise distribution
| T stage (number of patients) | Percentage |
|---|---|
| T1 (06) | 16.7 |
| T2 (28) | 17.85 |
| T3 (11) | 18.18 |
| T4 (28) | 25 |
Among the pathologically node positive group, (pN+), the median number of positive nodes was two (range 1–21). The level wise distribution of nodes was available for 108 of 113 patients. Level I was the most common level involved in pN+ group (67.8 %). Level II was involved in 17.5 % of pN+ patients. The overall involvement of level IV and V was 9.4 % and 4.7 % respectively. This nodal involvement at levels IV and V was always associated with positive nodes at level I or II. There was no case of skip metastasis occurring to either level IV or level V. The incidence of skip metastases to level III was merely 3.9 %. Positive nodes were found in more than one level in 35.2 % of patients in pN+ group. The positive lymph node percentage was 33.3 % and 44.7 % in early (T1 and T2) and late T stages (T3 and T4), respectively.
Discussion
Crile advocated radical neck dissection as essential component in management of head and neck cancers in 1906 [3]. Even though oral cancers were a minority in his series, RND became standard of treatment for all oral cancers. Over the years, the radicalism of RND has given way to MND, which by itself stands challenged by selective node dissections e.g. SOHND and of late the super selective lymph node dissection using sentinel lymph node biopsy (SLNB). However, despite years of clinical experience, technological and radiologic advances and multiple surgical procedures, a clear guideline that would ensure low failure rates without over treating the patients, still eludes us.
Clinically all patients can be grouped in to neck node negative (N0) or positive groups (N+). For a N0 neck, a difference in opinion exists between authors advocating wait and watch policy and those advocating a prophylactic therapy in high-risk group. Jalisi et al demonstrated 55 % survival in the neck dissection group as compared to 33 % in observation group. The locoregional control increased from 50 % to 91 % as well, when neck dissection was performed [4] as compared to the observation group. The salvage therapy often needed in the observation group was found to be more extensive than the elective therapy. This had lead to a consensus on elective therapy for patients with risk of occult metastases more than 20–25 %. A number of anatomic, radiographic, pathologic and clinical studies have demonstrated that lymphatic drainage of oral cancers occurs in a systematic and predictable manner [5]. It has been demonstrated that level IIb involvement in this setting is rare except in nasopharyngeal carcinoma [5]. Shah et al demonstrated only 9 % incidence of positive nodes in level IV [6]. The incidence of level V nodes was mere 2 % [6]. All the above observations make a Supraomohyoid Neck dissection (SOHND) an ideal candidate in node negative patients. Patients having pathologically negative nodes after SOHND have shown a failure of 10 % only [7]. In patients with pathologically positive nodes, failure rates ranges from 10 to 24 % [7]. The use of adjuvant RT in this setting brings down the rates to 0–15 % [7]. SOHND helps in preservation of injuries to important neck structures including spinal accessory hence providing best functional result with adequate nodal clearance in node negative patients Table 3.
The present recommendations advocate a modified radical neck dissection (MND) in all patients with positive neck nodes [7]. In fact, a comprehensive neck dissection is thought to be the only prudent surgical option by many for these patients [7, 8]. The rationale behind this recommendation is that even palpable nodes less than 3 cm in size have increased incidence of extracapsular spread. This may breach aponeurotic planes, which are preserved in MND II AND III. In our setup majority patients present with advanced stage disease (73.9 % T3 and T4 tumors in our series) with large, infected growths and hence the 75 % node positive rate in our patient group. However, a majority of positive neck patients eventually turn up to be pathologically node negative, (57.4 % patients in our group). In addition, the mean value for number of positive nodes in our series is only two. There is growing evidence in literature that SND can be employed in some of these patients as an alternative to MND I. Even though the rate of metastasis to all levels increases in patients with N+ disease, the pattern of involvement is still similar to what is seen in N0 neck [6]. Several authors have reported similar control rates with selective node dissection in the N+ neck [9, 10]. Andersen et al reported a 94.3 % regional control rates in node positive patients undergoing a SND [11]. Kowalski et al did not find isolated pN+ nodes at level IV or V in their analysis of N+ patients, results similar to this study. Hence if the nodes at level I and II are negative, a comprehensive neck dissection may not be needed. Their reported pN0 rate was 57.4 % in this subset of patients, which is similar to our rate [12]. Chepeha et al reported comparable regional control rates in N+ patients undergoing SND followed by RT as compared to patients subjected to MND with RT [13]. Medina et al reported a 12.5 % regional failure rate in N+ patients undergoing SND and RT [14]. The above studies do support an expanded role of SND in N+ patients. This is important in light of the fact that shoulder function is significantly worse in patients after MND as compared to SND [15]. A large prospective study is required to compare MND with SND in the N+ patients to conclusively establish SND as an alternative to MND but at present SND is still recommended for nodal disease present in levels I and II only [5].
Our results show that most of the patients in our setup present in advanced stages of the disease with large and often neglected oral ulcers. These patients tend to have poor oral hygiene with ulcerated growth and super added component of infection. This could be one of the reasons of high prevalence of significantly enlarged, clinically palpable nodes in our setup, which are eventually reported as reactive hyperplasia. The high prevalence of other co-existing granulomatous infections like tuberculosis can also add to ambiguity of the palpable nodes. The incidence of co-existing granulomatous infection is 3 % in all neck dissection specimens over the entire spectrum of OSCC in our database.
Current imaging studies are not reliable enough to base neck dissection decisions on their findings. The clinical examination is still the widely used method to classify patients into N0 or N+. However, it has proven inadequate for the same by many authors with approximately 40 % error rates [16]. Clinical findings can be supplemented by imaging techniques, but they too have fallen short of expectations. Ultrasound is an easily available; affordable but highly operator dependent tool [17] that has been advocated by some authors for guided FNAC to augment accuracy [18]. CT and MR mainly rely on the size of the node along with other criteria like necrosis to assess the nodal involvement but size in itself is not pathognomonic for involvement, with a high error rate if used as the sole criteria to establish involvement [19]. These investigations also add further to the cost of treatment, which may not be possible in a resource-starved setup. PET and PET-CT have shown promising results but the cost at present is too high to become a standard investigation in every patient. SLNB is another option that can improve accuracy of nodal staging while avoiding over treating this patient population but is still being investigated by larger studies to determine its exact role [20, 21].
Conclusion
The current methods of evaluation (clinical and imaging) are insufficient to reliably guide neck dissection decisions for buccal and alveolo-buccal cancer. The existing evidence, including this study, points to an increasing role of selective neck dissections over blanket use of comprehensive neck dissection in all patients with clinically palpable neck nodes.
Skip metastasis were rare in this subset, with no isolated involvement of level IV or V seen in our study. The pattern of nodal involvement seen hints at feasibility of selective neck dissection, especially when the level I and II nodes are negative. This would enable majority of patients to avoid comprehensive neck dissection and its associated morbidities. There is an urgent need to device more accurate methods of assessment of neck nodes and the need for prospective RCT for selective versus comprehensive neck dissection in this subset of patients.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Three year report of population based cancer registry, 2006–2008. Bangalore, India: Indian Council of Medical Research; 2010. [Google Scholar]
- 2.Ghosal S, Mallick I, Panda N, Sharma SC. Carcinoma of the buccal mucosa: Analysis of clinical presentation, outcome and prognostic factors. Oral Onco. 2006;42:533–539. doi: 10.1016/j.oraloncology.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Crile G. Excision of cancer of the head and neck with special reference to the plan of dissection based on one hundred and thirty-two operations. JAMA. 1906;47:1870. doi: 10.1001/jama.258.22.3286. [DOI] [PubMed] [Google Scholar]
- 4.Jalisi S. Management of the clinically negative neck in early squamous cell carcinoma of the zzoral cavity. Otolaryngol Clin North Am. 2005;38:37. doi: 10.1016/j.otc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Ferlito A, Rinaldo A, Silver CE, Gourin CG, et al. Elective and therapeutic selective neck dissection. Oral oncology. 2006;42:14–25. doi: 10.1016/j.ooe.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastasis from squamous cell carcinoma of the oral cavity. Cancer. 1990;66:109. doi: 10.1002/1097-0142(19900701)66:1<109::AID-CNCR2820660120>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Carlson ER, Smith B. Neck dissections for Oral/Head and neck cancer:1906–2006. J Oral Maxillofac Surg. 2006;64:4–11. doi: 10.1016/j.joms.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Shah JP, Anderson PE. Evolving role of modifications in neck dissection for oral squamous carcinoma. Br J Maxillofac Surg. 1995;33:3. doi: 10.1016/0266-4356(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 9.Byers RM. Modified neck dissection: a study of 967 cases from 1970 to 1980. Am J Surg. 1985;150:414–421. doi: 10.1016/0002-9610(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 10.Traynor S, Cohen J, Andersen P, Everts F. Results of selective neck dissection in the clinically positive neck. Am J Surg. 1996;172:654–657. doi: 10.1016/S0002-9610(96)00296-6. [DOI] [PubMed] [Google Scholar]
- 11.Andersen PE, Warren F, Bumingham A, Wong R, et al. Results of selective neck dissection in management of the node positive neck. Arch Otolaryngol Head Neck Surg. 2002;128:1180–1184. doi: 10.1001/archotol.128.10.1180. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski LP, Carvalho AL. Feasibility of supraomohyoid neck dissection in N1 and N2a oral patients. Head Neck. 2002;24:921–924. doi: 10.1002/hed.10127. [DOI] [PubMed] [Google Scholar]
- 13.Chepeha DB, Hoff PT, Taylor RJ, Bradford CR, Teknos TN, Esclamado RM. Selective neck dissection for the treatment of neck metastasis from squamous cell carcinoma of the head and neck. Laryngoscope. 2002;112:434–438. doi: 10.1097/00005537-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Medina JE, Byers RM. Supraomohyoid neck dissection: rationale, indications, and surgical technique. Head Neck. 1989;11:111–122. doi: 10.1002/hed.2880110203. [DOI] [PubMed] [Google Scholar]
- 15.Chepeha DB, Taylor RJ, Chepeha JC, Teknos TN, Bradford PK, Sharma PK, et al. Functional assessment using Constant’s Shoulder Scale after modified radical and selective neck dissection. Head Neck. 2002;24:432–436. doi: 10.1002/hed.10067. [DOI] [PubMed] [Google Scholar]
- 16.Teichgraber JF, Clairemont AA. Incidence of occult metastases for cancer of the oral tongue and floor of mouth: Treatment rationale. Head Neck Surg 1084;166:395–398 [DOI] [PubMed]
- 17.John DJ, Williams SR, Ahuja A, et al. Palpation compared with ultrasound in the assessment of malignant cervical lymph nodes. J Otol Laryngol. 1993;107:821–823. doi: 10.1017/s002221510012451x. [DOI] [PubMed] [Google Scholar]
- 18.Takes RP, Righi P, Meeuwis CA, et al. The value of ultrasound with ultrasound-guided fine needle aspiration biopsy compared to computed tomography in the detection of regional metastases in the clinical negative neck. Int J Radiat Oncol Biol Phys. 1998;40:1027–1032. doi: 10.1016/S0360-3016(97)00953-X. [DOI] [PubMed] [Google Scholar]
- 19.Som PM. Detection of metastases in cervical lymph nodes: CT and MR criteria and differential diagnosis. Am J Roentgenol. 1992;158:9619. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 20.Asthana S, Deo SS, Shukla NK, Jain P, Anand M, Kumar R. Intraoperative neck staging using sentinel node biopsy and imprint cytology in oral cancer. Head & Neck. 2003;25:368–372. doi: 10.1002/hed.10211. [DOI] [PubMed] [Google Scholar]
- 21.Alkureishi LWT, Burak Z, Alvarez JA, et al. Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma. Ann Surg Oncol. 2009;16:3190–3210. doi: 10.1245/s10434-009-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]