Figure 3.
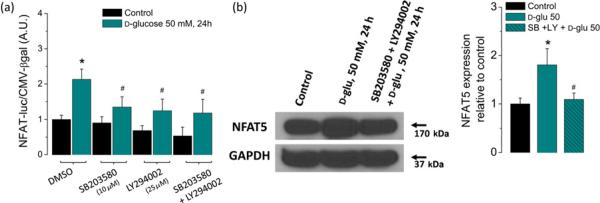
High glucose-dependent activation of the NFAT-luciferase reporter and NFAT5 expression are sensitive to inhibitors of stress kinases p38α and PIKK. FDB fibers were transfected as in Figure 1a. One day after transfection, fibers were transferred to isotonic or high-glucose (50 mmol/L) media with DMSO (0.5% v/v) or with SB203580 (10 μmol/L; a p38α inhibitor), LY2940002 (25 μmol/L; a PIKK inhibitor) or both (SB203580 + LY2940002) during 24 h. (a) Luciferase activity driven by NFAT normalized to β-galactosidase activity driven by CMV relative to control fibers. Mean ± S.E of three independent experiments is shown. (b) Western blot analysis of whole cell homogenates prepared from FDB fibers cultured in control isotonic media or in high d-glucose (50 mmol/L) media with DMSO (0.5% v/v) or with the combination of SB203580 + LY2940002 for 24 h by using NFAT5 antibody. The blot is representative of three independent experiments (three mice per group). The bar plot indicates that the increase on NFAT5 expression induced by elevated glucose is sensitive to inhibitors of stress kinases p38α and PIKK. *Indicates P< 0.05 compared with control; #P< 0.05 compared with fibers exposed to d-glucose without inhibitors. NFAT, nuclear factor of activated T-cell; DMSO, dimethyl sulfoxide; FDB, flexor digitorum brevis; PIKK, phosphoinositide 3-kinase-related kinase; CMV, cytomegalovirus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (A color version of this figure is available in the online journal)