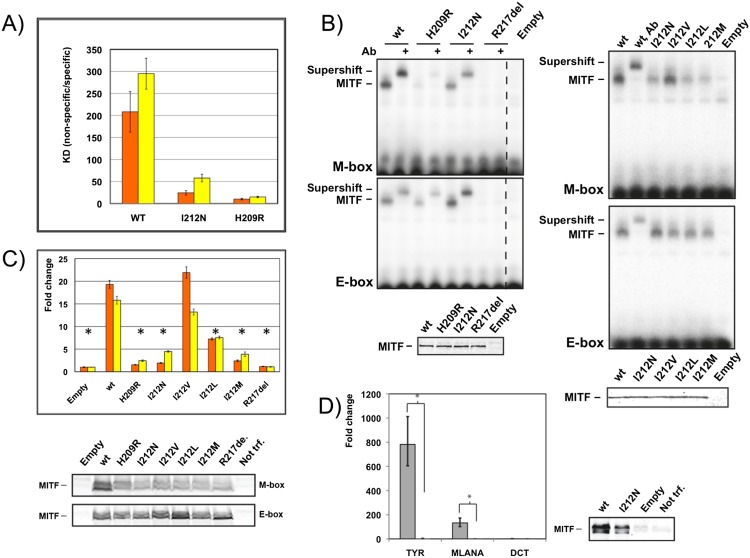
Figure 6.
Residue Ile212 is crucial for binding of MITF to the M-box. (A) Ratio of binding dissociation constants of different MITF variants to nonspecific DNA/M-box (orange) and nonspecific DNA/E-box (yellow) based on quantitative ITC experiments (Table 1). Error bars correspond to standard deviations, taking into account error propagation, as described in the Materials and Methods. (B) EMSAs showing altered M-box and E-box DNA binding of mutant MITF homodimer variants, as indicated. Supershifts with a MITF-specific antibody are indicated, confirming the specificity of the gel shifts. Western blots show equal loading of the respective proteins. (C) TA assays in HEK293T cells in which the same MITF mutants as in B were cotransfected together with TYR promoters containing either two M-boxes (orange) or E-boxes (yellow) as reporter. Data are represented as the standard error of the mean (SEM); (*) P < 0.05 based on an unpaired two-sided t-test. Western blots show levels of wild-type (wt) and mutant MITF proteins. (D) HEK293T cells transiently transfected with either MITF or MITF I212N were assayed for TYR, MLANA, and DCT expression (as control) by qPCR using the same error analysis as in C. Western blots show levels of wild-type and mutant MITF proteins. As the expression level of the MITF I212N mutant does not completely reach that of the wild-type protein, we consider the level of estimated amplification of expression for TYR and MLANA as the upper limit.