FIGURE 1:
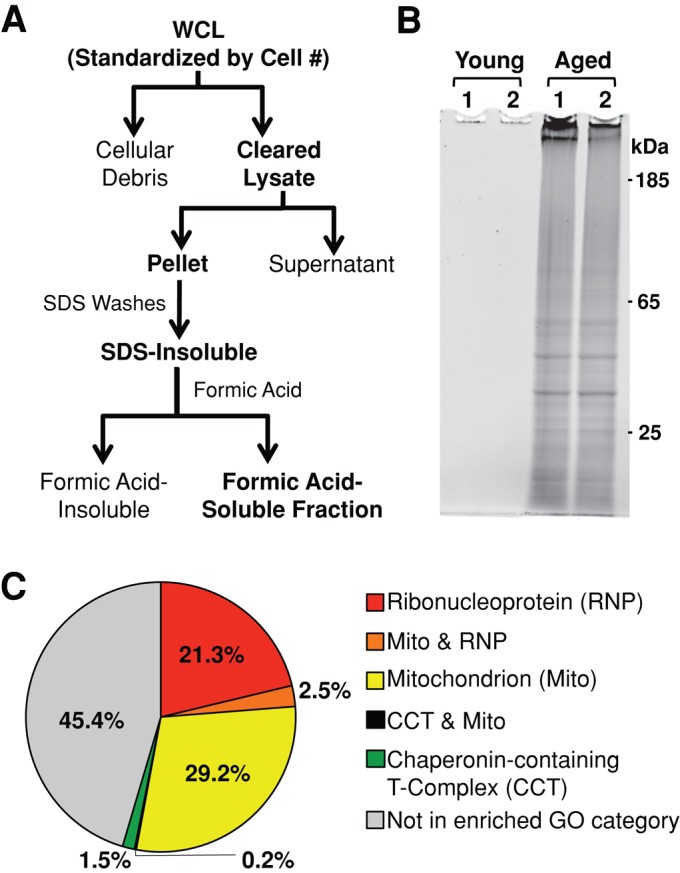
Insoluble proteins accumulate in chronologically aged S. cerevisiae. (A) Whole-cell lysates (WCL) were cleared of cellular debris via a low-speed centrifugation. Insoluble protein was pelleted from cleared lysates via high-speed centrifugation, washed with 1% SDS, and resolubilized in 70% formic acid. Formic acid was removed via desiccation, and remaining protein was resuspended in buffers compatible with downstream proteomic analysis. (B) Cells that had just entered the postmitotic state were harvested (young) or aged for 48 h postmitotically (aged) before preparation of insoluble protein. Formic acid–soluble protein was resuspended in loading buffer, run on polyacrylamide gels, and stained for total protein. Two biological replicates of each time point are shown (1, 2). SDS-insoluble protein from 8 × 107 cells was loaded in each lane. (C) GO analysis of the 480 SDS-insoluble proteins identified in aged cells revealed three significantly enriched cellular component GO categories: mitochondrion (GO:0005739), ribonucleoprotein complex (GO:0030529), and chaperonin-containing T-complex (GO:0005832).
