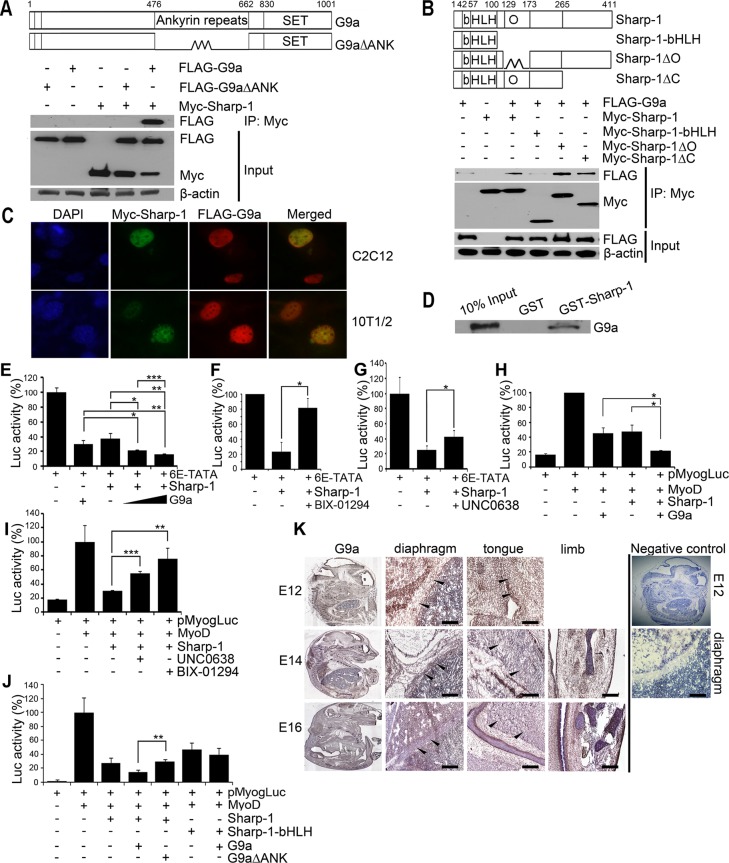
FIGURE 1:
G9a interacts with and enhances Sharp-1–mediated transcriptional repression. (A) Schematic representation of full-length G9a and a deletion mutant lacking the ankyrin repeats (G9a∆ANK). Numbers indicate amino acid residues. Cells transfected with Myc–Sharp-1, FLAG-G9a, and FLAG-G9a∆ANK were immunoprecipitated with anti–c-Myc agarose beads and immunoblotted with anti-FLAG antibody. Lysates were analyzed for Sharp-1 and G9a expression by Western blot. β-Actin was used as a loading control. (B) Schematic representation of Sharp-1 with basic (b), helix-loop-helix (HLH), and Orange (O) domains. Deletion mutants of Sharp-1 are shown. Sharp-1 was immunoprecipitated and analyzed for interaction with G9a using anti-FLAG antibody. (C) Colocalization of Myc–Sharp-1(green) and FLAG-G9a (red) was visualized by immunofluorescence in C2C12 and 10T1/2 cells. Nuclei were stained with DAPI (blue). (D) In vitro–translated G9a was used for interaction with equivalent amounts of GST–Sharp-1 and GST proteins. A 10% input was run as a control. (E–G) 10T1/2 cells were transfected with 6E-TATA-Luc along with Sharp-1 and G9a (E), with Myc-Sharp-1 and 2.5 μM of BIX-01294 (F), or with 0.25 μM UNC0638 (G). (H, I) Cells were transfected with pMyog-Luc, MyoD, Sharp-1, and G9a (H) and, in the presence of MyoD, Sharp-1 with 2.5 μM BIX-01294 or 0.25 μM UNC0638 (I). Error bars indicate mean ± SD. (J) Cells were transfected with pMyogLuc along with Sharp-1 and deletion mutants together with G9a and G9a∆ANK. Error bars indicate mean ± SD. (K) Mouse embryo sections were immunostained with anti-G9a antibody at E12, E14, and E16. Arrowheads in magnified images indicate G9a expression (brown staining) in diaphragm, tongue, and limb muscles. Negative control shows E12 embryo section stained with secondary antibody only. A magnified image of the diaphragm is shown. Scale bar, 100 μm.