Abstract
The blood-testis barrier (BTB) provides an efficient barrier to restrict paracellular and transcellular transport of substances, such as toxicants and drugs, limiting their entry to the testis to cause injury. This is achieved by the coordinated actions of efflux and influx transporters at the BTB, which are integral membrane proteins that interact with their substrates, such as drugs and toxicants. An efflux transporter (e.g., P-glycoprotein) can either restrict the entry of drugs/toxicants into the testis or actively pump drugs/toxicants out of Sertoli and/or germ cells if they have entered the seminiferous epithelium via influx pumps. This thus provides an effective mechanism to safeguard spermatogenesis. Using Sertoli cells cultured in vitro with an established tight junction (TJ)-permeability barrier which mimicked the BTB in vivo and treated with cadmium chloride (CdCl2), and also in adult rats (~300 g b.w.) treated with CdCl2 (3 mg/kg b.w., via i.p.) to induce testicular injury, cadmium was found to significantly downregulate the expression of efflux (e.g., P-glycoprotein, Mrp1, Abcg1) and influx (e.g., Oatp3, Slc15a1, Scl39a8) transporters. For instance, treatment of Sertoli cells with cadmium induced significant loss of P-glycoprotein and Oatp-3 at the cell-cell interface, which likely facilitated cadmium entry into the Sertoli cell. These findings illustrate that one of the mechanisms by which cadmium enters the testis is mediated by downregulating the expression of drug transporters at the BTB. Furthermore, cytokines and steroids were found to have differential effects in regulating the expression of drug transporters. Summary, the expression of drug transporters in the testis is regulated by toxicants, steroids and cytokines.
Keywords: testis, spermatogenesis, cadmium, environmental toxicant, drug transporters, P-glycoprotein, efflux drug pump, influx drug pump, Sertoli cell, blood-testis barrier
Introduction
Cadmium is one of the few environmental toxicants that has significant adverse side-effects in humans, in particular men’s reproductive health. It is also a known carcinogen. Cadmium has an unusual long half-life (~15–20 y) and it has a widespread presence in the ecosystem and food chains because industrial activities.1-7 However, its mechanism(s) of action remains largely unknown even though there are indications that cadmium exerts its effect at cell adhesion proteins, such as E-cadherin,8-10 causing kidney and endothelial tight junction (TJ)-barrier dysfunction.1,3,5,11 Cadmium also promotes carcinogenesis in lung, breast, kidney, prostate, stomach, pancreas, liver, head and neck, bladder, and testis, by perturbing cellular DNA repair mechanism, inducing DNA-protein cross-linking and cell proliferation, disrupting gene expression, and inhibiting cell apoptosis.7,12-15 It is known that in the mouse testis, drug transporter, Slc39a8 (solute carrier family 39, zinc transporter, member 8, also known as ZIP8), an influx pump, is the transporter responsible for cadmium-induced toxicity in the testis,16 illustrating cadmium enters multiple organs via the Slc39a8 transporter at the blood-tissue barriers in multiple organs, including the blood-testis barrier (BTB) in the testis. However, it is not known if cadmium can modulate the expression of drug transporters at the blood-tissue barriers, such as the BTB.
P-glycoprotein (P-gp, also known as multidrug resistance protein 1, Mdr1; or ATP-binding cassette sub-family B member 1, Abcb 1) and Mrp1 (multidrug resistance-related protein 1 also known as multidrug resistance-associated protein 1 and Abcc1) are ATP-binding cassette (ABC)-transporters, both are integral membrane proteins serving as efflux drug pumps that either pump drugs out of an epithelial or endothelial cell or prevent drugs from entering a cell via an ATP-dependent mechanism.7,17 Abcg1 (ATP-binding cassette sub-family G member 1) is a homolog of the Drosophila gene white,18 it is also an intracellular ATP-dependent efflux sterol transporter to facilitate the transport of cellular sterols to exogenous high-density lipoprotein (HDL).19 Several efflux drug transporters have recently been shown to be components of the BTB in the rat testis. For instance, P-glycoprotein was found to structurally interact with TJ-integral membrane proteins occludin, claudin-11 and JAM-A.20
Oatp3 (organic anion transporting polypeptide protein 3, also known as Slco1a5, solute carrier organic anion transporter family member 1a5) is an influx pump that transports thyroid hormones and prostaglandin E2 in mouse brain.21 Oatp3 was recently shown to structurally associate with N-cadherin, β-catenin, and ZO-1, but not occludin, at the BTB in the rat testis,22 illustrating these influx pumps together with the efflux pumps (e.g., P-glycoprotein) determine how much toxicants (e.g., cadmium) can “enter” the adluminal compartment of the seminiferous epithelium to induce testicular injury. Solute carrier family 15 member 1 (Slc15a1), also known as peptide transporter-1 (PepT-1), is an influx pump involved in oligopeptide reabsorption in the kidney and the intestines.23 Slc15a1 also facilitates the absorption of many peptidomimetic drugs in humans.24 Slc39a8, on the other hand, is also an influx drug pump, but earlier studies have shown that it is involved specifically in the transport of heavy metals, such as zinc25,26 and cadmium,16 across the blood-tissue barriers including the BTB. Unlike the ABC transporters, these influx pumps transport drugs across the blood-tissue barriers without involving the use of ATP.17,27
In light of the presence of several efflux and influx drug transporters at the BTB in the rat testes and their association with cell adhesion protein complexes at the BTB (e.g., occludin-ZO-1, N-cadherin-β-catenin, JAM-A-ZO-1, claudin-11-ZO-1),20,22 we sought to examine herein if cadmium-induced testicular toxicity would perturb the expression of these drug transporters, thereby facilitating or modifying their entry efficacy into the seminiferous epithelium to induce testis injury. Since cytokines (e.g., TGF-βs, TNFα, IL-1α)28-32 and steroids (e.g., testosterone, estradiol-17β) were shown to modulate BTB function,7,33,34 we also sought to examine if cytokines and steroids would modulate the expression of drug transporters in the testis.
Results
Cadmium downregulates the expression of efflux and influx drug transporters at the Sertoli cell BTB in vitro
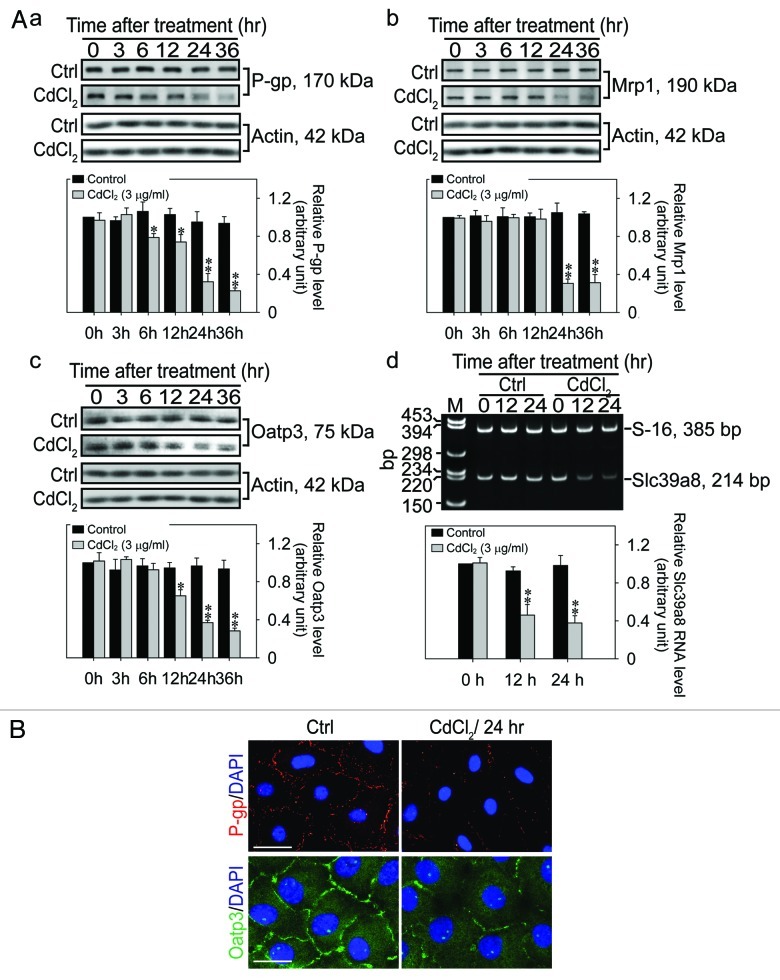
Sertoli cells cultured in vitro were known to establish a functional TJ-permeability barrier, and with the ultrastructures of TJ, basal ES, and gap junction as well as desmosome visibly by electron microscopy that mimicked the BTB in vivo.35-37 In fact, this in vitro system was widely used in the field by investigators to study Sertoli cell BTB dynamics.31,38-40 We thus used this system to assess if exposure of Sertoli cells to cadmium would lead to changes in the expression of several drug transporters including the putative cadmium influx transporter Slc39a8 (ZIP8) by immunoblotting and/or RT-PCR (Fig. 1). After Sertoli cells were cultured alone for 4 d when a functional TJ-permeability barrier was established,41,42 mimicking the Sertoli cell BTB in vivo,43,44 these cultures (designated time 0 h) were exposed to CdCl2 at 3 μg/ml (15 μM) and terminated at specified time points between 3 and 36 h for either immunoblotting (see Table 1) or RT-PCR (see Table 2) (since a working antibody against Slc39a8 was not available) vs. time 0, it was noted that cadmium significantly downregulated the expression of drug efflux pumps (e.g., P-glycoprotein, Mrp1), and drug influx pumps (e.g., Oatp3, Slc39a8) (Fig. 1A, a−d). These findings support the notion that the unusual sensitivity of the testis to cadmium-induced injury, such as the BTB,45-47 is due to a downregulation of drug transporters at the BTB by cadmium, as such, cadmium has unlimited access to the seminiferous epithelium behind the BTB. The findings shown in Figure 1A (panels a–d) depicting downregulation of drug transporters by cadmium were supported by studies using immunofluorescence microscopy as shown in Figure 1B, illustrating considerable loss of both P-glycoprotein and Oatp3 at the Sertoli cell-cell interface following exposure of these Sertoli cells to CdCl2 for 24 h, possibly via an increase in endocytic vesicle-mediated endocytosis and intracellular degradation of these drug transporters.
Figure 1. Effects of cadmium on the expression of drug transporters in Sertoli cell epithelium with an established functional TJ-permeability barrier in vitro. (A) Sertoli cells cultured alone for 4 d at 0.5 x 106 cells/cm2 with an established functional TJ-permeability barrier were treated with CdCl2 (Mr 183.32) at 3 μg/ml (~15 μM) or without (control, Ctrl) for 0–36 h and cultures were terminated at specified time points. The steady-state protein or mRNA levels of P-glycoprotein (P-gp) (a), Mrp1 (b), Oatp3 (c) and Slc39a8 (d) were then quantified by immunoblottings (a–c) or RT-PCR (d) since a satisfactory antibody was not available for Slc39a8. The lower panels in (a–d) are the histograms of the corresponding immunoblotting or RT-PCR data using data shown in the upper panels from n = 3 experiments from different Sertoli cell cultures. *p < 0.05; **p < 0.01. (B) Sertoli cells cultured at 0.05 × 106 cells/cm2 were treated with CdCl2 for 24 h and stained for P-gp (red fluorescence) or Oatp3 (green fluorescence) vs. the control control (Ctrl) with Sertoli cell nuclei stained by DAPI. Bar = 40 μm and 20 μm for P-gp and Oatp3 in the left panel, which apply to the micrographs in the right panel.
Table 1. Primary antibodies used for different experiments in this report*.
| Antibody | Catalog # | Lot # | Host | Vendor | Working dilution | |
|---|---|---|---|---|---|---|
| |
|
|
|
|
IB |
IF |
|
P-glycoprotein |
sc-55510 517310 |
B1108 D00022523 |
Mouse Mouse |
Santa Cruz Biotechnology Calbiochem |
1:200 1:250 |
1:50 |
|
Mrp1 |
sc-13960 |
I1906 |
Rabbit |
Santa Cruz Biotechnology |
1:200 |
|
|
Abcg1 |
ab52617 |
467040 |
Rabbit |
Abcam |
1:2000 |
|
|
Oatp3 |
sc-47265 |
A1408 |
Goat |
Santa Cruz Biotechnology |
1:200 |
1:50 |
|
Slc15a1 |
LS-C18855 |
12477 |
Rabbit |
Lifespan Biosciences |
1:1000 |
|
| Actin | sc-1616 | F0809 | Goat | Santa Cruz Biotechnology | 1:200 | |
Antibodies used herein were shown to cross-react with their corresponding proteins in rats as indicated by the manufacturers. IB, immunoblotting; IF, immunofluorescence microscopy.
Table 2. Primers used for RT-PCR experiments in this report.
| Gene | Primer sequence | Orientation | Position | Length (b.p.) | Tm (°C) | Cycle no. | GenBank accession number |
|---|---|---|---|---|---|---|---|
|
Slc39a8 |
5′- AGTTGCTGTGTTTGGTGG-3′ 5′- GTGTCCATTAGGCTCAGTG-3′ |
Sense Antisense |
597–614 792–810 |
214 |
53 |
25 |
BC089844 |
| S16 | 5′-TCCGCTGCAGTCCGTTCAAGTCTT-3′ 5′-GCCAAACTTCTTGGTTTCGCAGCG-3′ |
Sense Antisense |
15–38 376–399 |
385 | 53 | 25 | XM_341815 |
b.p., base pairs; Tm, annealing temperature
Cadmium downregulates the expression of efflux and influx drug transporters in the rat testis in vivo.
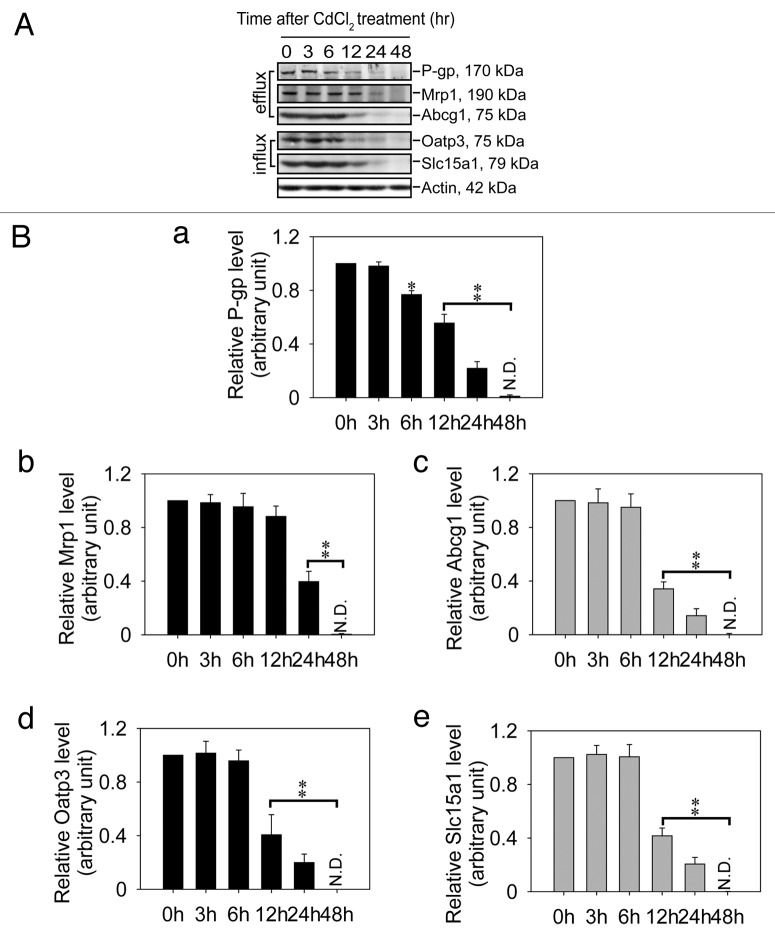
Downregulation of drug transporters in Sertoli cells at the BTB following their exposure to cadmium in vitro as shown in Figure 1 was also consistent with findings in vivo when the steady-state protein levels of efflux pumps (e.g., P-glycoprotein, Mrp1, Abcg1) and influx pumps (e.g., Oatp3, Slc15a1) were examined by immunoblotting (Fig. 2A). A downregulation of drug transporters by CdCl2 in the rat testis by as much as ~5- to 10-fold was detected when these blots were densitometrically scanned and compared statistically as shown in (Fig. 2B), illustrating the findings using the in vitro system to study Sertoli cell BTB function that demonstrated the disruptive effects of cadmium on the expression of these efflux and influx transporters are physiologically relevant.
Figure 2. Effects of cadmium on the expression of drug transporters in the rat testis during cadmium mediated testicular injury. Groups of adult rats (n = 3 rats for each time point including time 0, which served as a control) at 250–300 g b.w. were treated with CdCl2 at time 0 via i.p. and 3 rats were terminated at specified time points. Lysates were prepared from these testes for immunoblotting as shown in (A) to quantify the steady-state levels of different efflux drug transporters: P-glycoprotein (P-gp), Mrp1, and Abcg1; and influx drug transporters: Oatp3 and Slc15a1 with 100 μg protein per lane using lysates from testes. Data from 3 rats such as those shown in (A) were scanned and normalized against actin which served as a loading control and the histograms are shown in (a–d) in (B) corresponding to the five drug transporters. N.D., not detectable; *p < 0.05; **p < 0.01.
Differential effects of steroids and cytokines on the expression of drug transporters in Sertoli cells in vitro
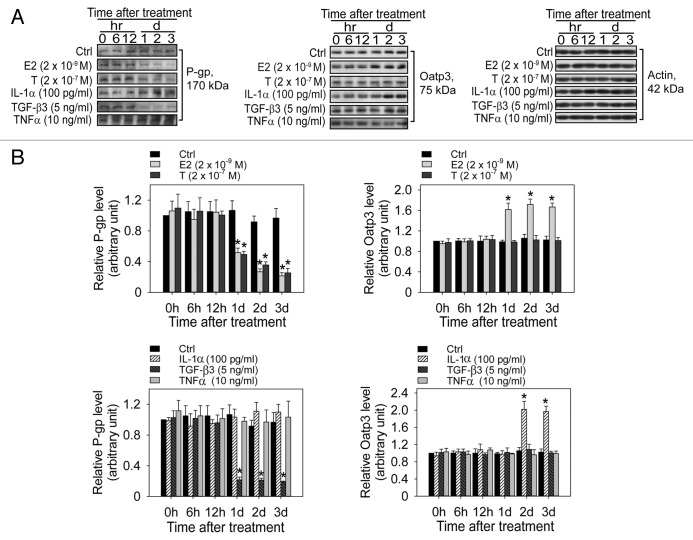
We next examined the effects of two other classes of biomolecules, namely steroids and cytokines, which are known to regulate Sertoli cell BTB function,7 if they would modulate the expression of the efflux transporter P-glycoprotein and the influx transporter Oatp3 (Fig. 3A and B). Steroids, such as estradiol-17β and testosterone; and cytokines, such as IL-1α, TGF-β3 and TNFα; were earlier found to modulate Sertoli cell BTB function either in vitro, in vivo or both.28,29,32,44,48-50 Interestingly, estradiol-17β, testosterone and TGF-β3 were shown to downregulate the expression of P-glycoprotein, whereas IL-1α and TNFα had no apparent consistent effects on P-glycoprotein expression (Fig. 3A and B). However, estradiol-17β and IL-1α upregulated Oatp3 but testosterone, TGF-β3 and TNFα apparently had no effects on Oatp3 expression (Fig. A and B). In short, these findings are in contrast to cadmium treatment since cadmium uniformly downregulated all the efflux and influx drug transporters that were examined but steroids and cytokines were shown to have differential effects on the expression of efflux transporter P-glycoprotein and influx drug transporter Oatp3 (Fig. 3 vs. Fig. 2).
Figure 3. Effects of steroids and cytokines on the expression of different drug transporters in Sertoli cells cultured in vitro with an established functional TJ-permeability barrier. (A) Steroids, such as estradiol-17β (E2) and testosterone (T); and cytokines, such as IL-1α, TGF-β3 and TNFα, at concentrations known to be found in the testis and were shown to regulate Sertoli cell function were added to the Sertoli cells cultured in vitro on day 4 when a functional TJ-permeability barrier that mimicked the BTB in vivo was established. At specified time points, these cultures were terminated from different treatment groups vs. the control (Ctrl) group, and lysates were obtained for immunoblotting (40 μg protein per lane)using antibodies against either P-glycoprotein (P-gp) (left panel) or Oatp3 (right panel) with actin (bottom panel) served as the loading control. (B) Histograms that summarize results of immunoblotting, such as those shown in (A) were densitometically scanned using SigmaGel and normalized against actin, and the steady-state protein level at time 0 was arbitrarily set at 1. Statistical analysis was performed by comparing the level of a drug transporter following treatment to the level of the corresponding control at each time point. *p < 0.01.
Discussion
In the mouse, Slc39a8 (also known as Zip8) was shown to be the influx drug pump responsible for transporting cadmium across the BTB to induce toxicity in the testis.16 However, studies have shown that other efflux and influx transporters may also be capable of transporting heavy metals, such as cadmium, into and out of the Sertoli and germ cells in the testis. Recent studies have shown that these drug transporters are not limited to the Sertoli cell, the nursery cell type in the seminiferous epithelium that provides structural supports, nutrients and other necessary biomolecules to the developing germ cells during spermatogenesis; instead, germ cells at various stages of their development express different types of efflux and influx drug transporters in the rat testis.17,22,51 Herein, it was shown that when Sertoli cells cultured in vitro with an established functional TJ-permeability barrier that mimics the BTB in vivo were exposed to cadmium, this toxicant can downregulate several efflux and influx drug transporters, illustrating that this toxicant is capable of dismantling the machinery that is put in place at the BTB to protect the unwanted entry of toxicants. The downregulation of these drug pumps by cadmium is not restricted to findings in vitro since the expression of several selected drug efflux and influx drug transporters was similarly downregulated when rats were exposed to cadmium in vivo. These findings were further validated by immunofluorescence microscopy when the expression and localization of P-glycoprotein and Oatp3 were examined before and after exposure to cadmium. These observations also support the notion that even though there is a sophisticated system in place in the testis involving both drug efflux and influx pumps to protect germ cell development behind the testis, and that this similar system is also in place in germ cells outside the BTB, such as in spermatogonia and early spermatocytes,7,51 certain toxicants, such as cadmium as shown herein, can disrupt their expression, so that there is no checkpoint in place in the testis to reduce their levels in the testis. However, it is not known at the moment if such a downregulation of drug transporters can impede the Sertoli cell TJ-permeability barrier function as demonstrated in earlier studies in which cadmium was found to perturb the Sertoli cell TJ-barrier.38,52,53 Recent studies, however, have shown that drug transporters, such as P-glycoprotein and Oatp3, are integrated components of the protein adhesion complexes that constitute the BTB, such as occludin-ZO-1, JAM-A-ZO-1, claudin-11-ZO-1 or N-cadherin-β-catenin.20,22,41 Thus, downregulation of the drug transporters that are part of these adhesion protein complexes may perturb the integrity of these proteins, thereby impeding their functionality in conferring the barrier function at the Sertoli cell BTB. This possibility warrants further studies to examine the role of drug transporters in blood-tissue barriers.
During spermatogenesis, steroids and cytokines play different functional roles to regulate spermatogenesis, which include germ cell survival and apoptosis, inflammatory responses, cell cycle progression, meiosis, cell adhesion function and BTB function.7,34,54-58 It is of interest to note that steroids and cytokines have differential effects on the expression of the efflux transporter P-glycoprotein and the influx transporter Oapt3. It is likely that these regulatory effects are also stage-specific depending on the cycle of the seminiferous epithelium. While in this report, we have limited our studies to examine the effects of steroids and cytokines on the expression of P-glycoprotein and Oatp3, it is highly likely that steroids and cytokines play a crucial role in regulating the expression of the complex network of drug transporters since dozens of drug transporters are present in the testis.7 The net result of these changes induced by steroids and cytokines determines the steady-state levels of different biomolecules, steroids, therapeutic drugs, paracrine/autocrine factors, and/or hormones that are available to developing germ cells and/or Sertoli cells in the seminiferous tubules both behind and outside the BTB during the epithelial cycle of spermatogenesis. For instance, it is now known that influx drug transporters, such as organic anion-transporting polypeptides (Oatps), regulate the uptake of steroids in breast epithelial and breast cancer cells,59 and efflux transporters (e.g., P-glycoprotein) also regulate the bioavailability of antiretroviral drugs across the BTB.60 Thus, our findings illustrate that endogenous steroids and cytokines in the testis can modulate the expression and thus the function of drug transporters, thereby modulating the levels of steroids and therapeutic drugs in the testis, perhaps altering the homeostasis of the testis in response to changes in the environment during normal and pathophysiological conditions. However, the phenotypic effects of the steroid- or cytokine-mediated expression of drug transporters on different testicular functions remain to be determined. It is likely that steroids and cytokines fine-tune the levels of different biomolecules in the seminiferous epithelium via their effects on drug transporters, so that the steady-state levels of various biomolecules in metabolically quiescent germ cells can be tightly regulated during spermatogenesis.
In summary, we have demonstrated herein the expression of drug transporters is regulated by environmental toxciants (e.g., cadmium), steroids (e.g., estradiol-17β, testosterone), and cytokines (e.g., TNFα, TGF-β3). Functional studies can now be designed to examine the functional significance of these findings.
Materials and Methods
Animals
Male Sprague-Dawley rats at 20 d of age, and adult male rats at ~250–300 g b.w. (body weight) were purchased from Charles River Laboratories. Rats had free access to water and standard rat chow, and they were maintained at 22°C with a 12:12 h of light:dark cycle. Rats were euthanized by CO2 asphyxiation. The use of animals for the experiments reported herein was approved by The Rockefeller University Animal Care and Use Committee with Protocol numbers 09016 and 12056.
Sertoli cell cultures
Sertoli cells were isolated from 20-d-old rat testes and plated on Matrigel™-coated 12-well plates at 0.5 × 106 cells/cm2 as earlier described.61 Sertoli cells were cultured in serum-free F12/DMEM with supplements and incubated at 35°C in a humidified atmosphere of 5% CO2/95% air [v/v]. About 36–48 h after cell plating, these cultures were subjected to a hypotonic treatment using 20 mM Tris (pH 7.4 at 22°C) for 2.5 min to lyse residual germ cells, and cultures were washed in F12/DMEM as described.62 The purity of these cultures is ~98% with negligible contaminations of Leydig cells, germ cells and/or peritubular myoid cells when specific markers for these cells were used and assessed by either RT-PCR or immunoblotting as described.63 Sertoli cells were cultured alone for 4-d before different treatments. Cells were terminated at specified time points thereafter for immunoblotting or RT-PCR. For fluorescence microscopy, Sertoli cells were plated on Matrigel-coated coverslips at 0.05 × 106 cells/cm2, and placed in 6-well dishes with 5-ml F12/DMEM per well. This cell density was selected for microscopy so that Sertoli cells were evenly spaced and protein localization at the cell-cell interface was easily detected. In experiments to be used to obtain lysates for immunoblotting or for nucleic acid extraction for RT-PCR, a Sertoli cell density of 0.5 × 106 cells/cm2 was used so that sufficient protein or RNA can be obtained from each well in the 12-well dishes (each well contained 3-ml F12/DMEM) for analysis. These experiments were repeated at least three times using different batches of Sertoli cells and each time point had triplicate cultures.
Treatment of Sertoli cells with cadmium, steroids and cytokines
On day 4 of culture when Sertoli cells were found to establish a functional TJ-permeability barrier in vitro based on the presence of a stable TER (transepithelial electrical resistance) across the Sertoli cell epithelium as described,22,41 cells were incubated with CdCl2 (Mr 183.32) at 3 μg/ml (~15 μM) for 0, 3, 6, 12, 24 and 36 h; estradiol-17β (E2, 2 × 10−9 M), testosterone (T, 2 × 10−7 M), IL-1α (100 pg/ml), TGF-β3 (5 ng/ml), or TNF-α (10 ng/ml) for 0, 6, 12 h and 1, 2, 3 d at 35°C. Corresponding vehicle controls were used without cadmium, steroids or cytokines in F12/DMEM.
Treatment of rats with cadmium
Adult rats were treated with CdCl2 dissolved in saline (20 mg/ml) at 3 mg/kg b.w., i.p., as described previously,46 which is known to disrupt the BTB irreversibly and induces germ cell loss from the epithelium.45-47 Rats were euthanized at different time points at 0, 3, 6, 12, 24 and 48 h after treatment with n = 3 rats per time point. Testes were immediately removed, frozen in liquid nitrogen and stored at -80°C until used for lysate preparation.
Immunoblot analysis
Testis or Sertoli cell lysates were prepared in lysis buffer [10 mM Tris, pH 7.4 at 22°C containing 0.15 M NaCl, 10% glycerol (v/v), 1% NP-40 (v/v)] with cocktails of protease inhibitors and phosphatase inhibitors from Sigma-Aldrich as described.22,41 Protein concentrations in samples were estimated by using BioRad DC (detergent compatible) Protein Assay kits and a BioRad Model 680 Spectrophotometer. Approximately 100 μg testis lysate or 40 μg Sertoli cell lysate per lane was resolved onto SDS-polyacrylamide gels under reducing conditions. Proteins in these gels were transferred onto nitrocellulose, blocked with 5% milk [w/v] in PBS-Tris [pH 7.4 containing 0.1% Tween-20 (v/v)] for 1 h, and probed with different primary antibodies at room temperature overnight. Blots were then incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology), and chemiluminescence was performed as described using a kit prepared in our laboratory64 to detect the target proteins. Images were captured with a LAS-4000 luminescent image analyzer (FujiFilm). Densitometric analysis was performed using SigmaGel.
RT-PCR
Since a specific anti-Slc39a8 antibody was not available for immunoblotting analysis, we had used RT-PCR to assess any changes on the steady-state mRNA level of Slc39a8 following cadmium treatment. Total RNAs were extracted from Sertoli cells after cadmium treatment for 0, 12 and 24 h using TRIzol reagent (Invitrogen). Contaminating genomic DNA in each RNA sample, if any, was digested with RNase-free DNase I (Invitrogen) prior to their use for reverse transcription into cDNAs using Moloney murine leukemia virus reverse transcriptase (M-MLV RT) reagent (Invitrogen) as described.20 PCR was performed using primer pair specific to target gene Slc39a8 (Table 2), which was co-amplified with ribosomal S16 serving as an internal control for equal sample processing and RNA loading.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed using primary Sertoli cells after 24 h-cadmium treatment. Sertoli cells were fixed in 4% paraformaldehyde [w/v], permeabilized with 0.1% Triton X-100 [v/v] and blocked with 1% BSA [w/v]. Thereafter, cells were incubated with either anti-P-glycoprotein or anti-Oatp3 diluted at 1:50 in 1% BSA at room temperature overnight. Cells were then washed and incubated with Alexa Fluor 555 (red color) or 488 (green color) secondary antibodies diluted at 1:100 in 1% BSA. ProLong Gold antifade reagent with DAPI was used for mounting, and images were acquired using an Olympus BX61 fluorescence microscope with a built-in Olympus DP71 digital camera at 12.5 MPx as described.41,65
Statistical analysis
All in vitro culture experiments reported herein were repeated at least three times with duplicate or triplicate wells using different batches of Sertoli cells. For in vivo experiments, at least three rats for each time point were used. Statistical analysis was performed with GB-STAT (Version 7.0, Dynamic Microsystems). Student’s t-test was used for paired comparisons against the control, whereas one-way ANOVA followed by Dunnett’s post-test was used for multiple comparisons.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034 to C.Y.C.), and National Natural Science Foundation of China (NSFC, Grant Number 31101043 to L.S.)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/22536
References
- 1.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–9. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mruk DD, Cheng CY. Environmental contaminants: Is male reproductive health at risk? Spermatogenesis. 2011;1:283–90. doi: 10.4161/spmg.1.4.18328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng CY, Wong EW, Lie PP, Li MW, Su L, Siu ER, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:1676–84. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong EWP, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–9. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 2010;56:147–67. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prozialeck WC, Lamar PC. Interaction of cadmium (Cd(2+)) with a 13-residue polypeptide analog of a putative calcium-binding motif of E-cadherin. Biochim Biophys Acta. 1999;1451:93–100. doi: 10.1016/S0167-4889(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 9.Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and β-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180–95. doi: 10.1016/S0041-008X(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 10.Prozialeck WC, Edwards JR. Cell adhesion molecules in chemically-induced renal injury. Pharmacol Ther. 2007;114:74–93. doi: 10.1016/j.pharmthera.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong EWP, et al. Cell junctions in the testis as targets for toxicants. In: Comprehensive Toxicology. 2nd Edition (McQueen, CA, Ed; Series Editor); Vol. 11 Reproductive and Endocrine Toxicology. Hoyer, P.B., Richburg, J.H. (Ed.); Oxford: Academic Press, Elseiver; pp. 167-188. (2010). [Google Scholar]
- 12.Luparello C, Sirchia R, Longo A. Cadmium as a transcriptional modulator in human cells. Crit Rev Toxicol. 2011;41:75–82. doi: 10.3109/10408444.2010.529104. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics. 2011;6:820–7. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khlifi R, Hamza-Chaffai A. Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: a review. Toxicol Appl Pharmacol. 2010;248:71–88. doi: 10.1016/j.taap.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Koedrith P, Seo YR. Advances in carcinogenic metal toxicity and potential molecular markers. Int J Mol Sci. 2011;12:9576–95. doi: 10.3390/ijms12129576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA. 2005;102:3401–6. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier, and spermatogenesis. J Endocrinol. 2011;208:207–23. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Rossier C, Lalioti MD, Lynn A, Chakravarti A, Perrin G, et al. Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am J Hum Genet. 1996;59:66–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc Natl Acad Sci USA. 2011;108:19719–24. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–87. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsuki S, Takizawa T, Takanaga HH. S., Hosoya, K. & Terasaki, T. Localization of organic anion transporting polypeptide 3 (oatp3) in mouse brain parencymal and cpillary endothelial cells. J Neurochem. 2004;90:743–9. doi: 10.1111/j.1471-4159.2004.02549.x. [DOI] [PubMed] [Google Scholar]
- 22.Su L, Mruk DD, Lee WM, Cheng CY. Drug transporters and blood--testis barrier function. J Endocrinol. 2011;209:337–51. doi: 10.1530/JOE-10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113:332–40. doi: 10.1016/S0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- 24.Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, et al. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995;270:6456–63. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- 25.Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2004;36:40–5. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 26.Besecker B, et al. The human zinc trasnporter SLC39A8 (Zip8) is critical in zinc-mediate cytoprotection in lung epithelia. Am J Physiol Lung Physiol. 2008;294:L1127–36. doi: 10.1152/ajplung.00057.2008. [DOI] [PubMed] [Google Scholar]
- 27.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–54. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lie PPY, Cheng CY, Mruk DD. Interleukin-1α is a regulator of the blood-testis barrier. FASEB J. 2011;25:1244–53. doi: 10.1096/fj.10-169995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–87. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 31.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–77. doi: 10.1210/en.142.5.1865. [DOI] [PubMed] [Google Scholar]
- 32.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–59. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30:119–32. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong CCS, Chung SS, Grima J, Zhu LJ, Mruk D, Lee WM, et al. Changes in the expression of junctional and nonjunctional complex component genes when inter-sertoli tight junctions are formed in vitro. J Androl. 2000;21:227–37. [PubMed] [Google Scholar]
- 36.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 37.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–29. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 38.Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures--a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–7. doi: 10.1016/0041-008X(92)90278-Z. [DOI] [PubMed] [Google Scholar]
- 39.Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl. 1996;17:249–55. [PubMed] [Google Scholar]
- 40.Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α 2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–40. doi: 10.1016/0303-7207(92)90219-V. [DOI] [PubMed] [Google Scholar]
- 41.Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci USA. 2011;108:19623–8. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L, Cheng CY, Mruk DD. Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:1864–75. doi: 10.1016/j.biocel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–86. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the ‘blood-testis barrier’ after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–6. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 46.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–98. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 47.Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–9. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 48.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, et al. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–33. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 50.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–33. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 52.Chung NPY, Cheng CY. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–88. doi: 10.1210/en.142.5.1878. [DOI] [PubMed] [Google Scholar]
- 53.Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis : chronic inflammation in an immunologically privileged tissue? Adv Exp Med Biol. 2008;636:92–114. doi: 10.1007/978-0-387-09597-4_6. [DOI] [PubMed] [Google Scholar]
- 55.Shaha C. Estrogens and spermatogenesis. Adv Exp Med Biol. 2008;636:42–64. doi: 10.1007/978-0-387-09597-4_3. [DOI] [PubMed] [Google Scholar]
- 56.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–35. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/er.22.3.289. [DOI] [PubMed] [Google Scholar]
- 58.Sharpe RM. Regulation of spermatogenesis. In: The Physiology of Reproduction. Eds. Knobil, E., Neill, J.D. New York, Raven Press. pp. 1363-1434 (1994). [Google Scholar]
- 59.Banerjee N, Allen C, Bendayan R. Differential role of organic anion-transporting polypeptides in estrone-3-sulphate uptake by breast epithelial cells and breast cancer cells. J Pharmacol Exp Ther. 2012;342:510–9. doi: 10.1124/jpet.112.192344. [DOI] [PubMed] [Google Scholar]
- 60.Robillard KR, Hoque T, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther. 2012;340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- 61.Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–52. doi: 10.1007/978-1-61779-191-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;2:249–54. [Google Scholar]
- 63.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–15. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 64.Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1:121–2. doi: 10.4161/spmg.1.2.16606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–60. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]