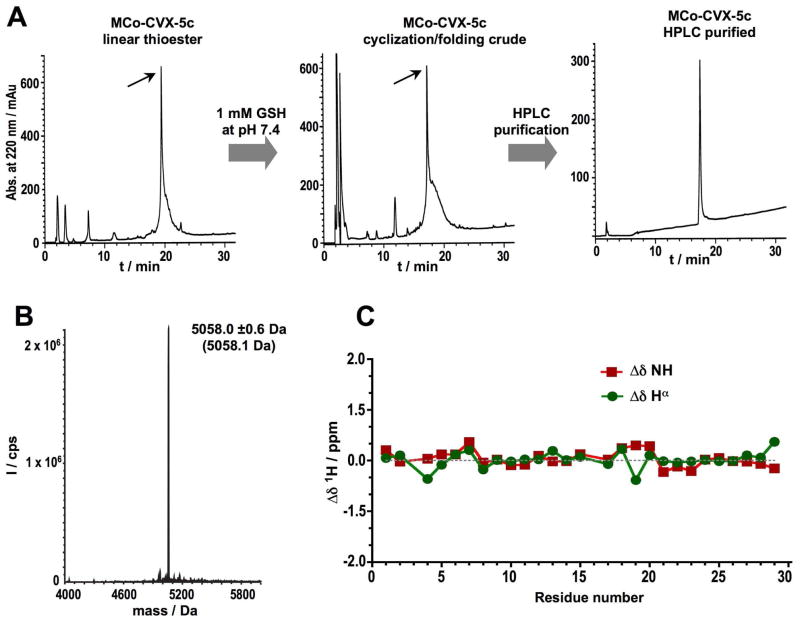
Figure 2.
Chemical synthesis and characterization of cyclotide MCo-CVX-5c. A. Analytical HPLC traces of the linear thioester precursor, GSH-induced cyclization/folding crude after 96 h and purified cyclotide. An arrow indicates the desired peptide. B. ES-MS characterization of pure MCo-CVX-5c. The expected average molecular weight is shown in parenthesis. C. Chemical shifts differences of the backbone, NH and Hα protons between the common sequence (residues 1 through 29) of MCoTI-I24, 25 and MCo-CVX-5c (Table S2).