Abstract
A unique non-laying strain of chickens with heritable hyperlipidemia and aortic atherosclerosis was first described in 1974. Subsequent work established that the phenotype results from a naturally occurring point mutation in the gene specifying the very low density lipoprotein (VLDL) receptor, a 95-kDa membrane protein which normally mediates the massive uptake of the main circulating hepatically-synthesized yolk precursors, VLDL and vitellogenin. As a result, hens of the mutant strain termed “restricted ovulator” (R/O) have approximately 5-fold elevations in circulating cholesterol and triglyceride concentrations compared with normal layers, and hepatic lipogenesis and cholesterogenesis are markedly attenuated due to feedback inhibition. R/O hens also exhibit hyperestrogenemia, hypoprogesteronemia, elevated circulating gonadotropins, and up-regulated pituitary progesterone receptor mRNA and isoforms. The ovaries of R/O hens are abnormal in that they lack a follicular hierarchy and contain many small preovulatory follicles of various colors, shapes, and sizes. However, since R/O hens occasionally lay eggs, it is possible that endocytic receptors other than the VLDL receptor may be able to facilitate oocyte growth and/or that yolk precursor uptake can occur via a nonspecific bulk process. A mammalian model of impaired fecundity with abnormal lipoprotein metabolism also has been described, but different mechanisms are likely responsible for its reproductive dysfunction. Nevertheless, as our understanding of the molecular physiology and biochemistry of avian oocyte growth continues to expand, in part due to studies of the R/O model, new analogies may emerge between avian and mammalian systems, which ultimately could help to answer important questions in reproductive biology.
Keywords: Chicken, Hyperlipidemia, Oocyte, Point mutation, Restricted ovulator, Very low density lipoprotein receptor
1. Introduction
A unique strain of hyperlipidemic female White Leghorn chickens that failed to lay eggs was initially discovered at DeKalb Agricultural Research, Inc. (DeKalb, IL) in the early 1970s. All of the hens were the daughters of a single rooster thought to carry a mutation responsible for the non-laying condition. The existence of this non-laying strain was first reported in the literature by Ho et al. (1974). As compared with wild-type (WT) hens, non-laying hens had approximately 5-fold higher serum levels of cholesterol and triglycerides, a slower daily turnover of cholesterol, attenuated endogenous cholesterol biosynthesis, developed aortic atherosclerosis, and exhibited abnormal ovarian morphology (Ho et al., 1974). In each of two separate matings of the original mutant DeKalb rooster with WT females, approximately one-half of the offspring hens failed to lay eggs upon reaching sexual maturity. Thus, the condition was heritable and it appeared that the rooster simply carried a mutation causing the abnormal female phenotype since his reproductive functions, secondary sex characteristics, and serum lipid levels were normal (discussed in Section 3). Hence, Ho et al. (1974) referred to the original rooster and those of subsequent generations as “carrier roosters”.
Jones et al. (1975) coined the term “restricted ovulator” (R/O) to describe females possessing the mutant gene. Two years later, McGibbon (1977) provided evidence that the sterile condition associated with spontaneous follicular involution was sex-linked and resulted from a single gene defect at a locus (ro) on the sex chromosome Z. In Aves, females are the heterogametic sex; thus, males are ZZ and hemizygous females are ZW (Hutt, 1964). Afflicted heterozygous (“carrier”) roosters therefore pass on the mutation in one of their Z chromosomes to one-half of their male and to one-half of their female progeny. Although it is theoretically possible to produce a homozygous mutant male by mating a carrier rooster to a mutant hen that is able to lay eggs, only heterozygous roosters possessing one WT allele and one mutant allele for the ro gene have been obtained for investigational purposes to date.
Here, we provide the first comprehensive review centered on the R/O chicken strain and describe a unique oviparous vertebrate model of reproductive dysfunction, which is uniquely positioned at the crossroad of ovarian follicle/oocyte development and lipoprotein metabolism. The authors’ interest in the R/O strain dates back to the late 1980s, when the laboratory of WJS first described the molecular defect responsible for this dramatically abnormal phenotype. Subsequent collaborative studies have further delineated various reproductive, metabolic, and physiological characteristics of this valuable and useful animal model.
The R/O strain was initially housed at DeKalb Agricultural Research (D.G. Jones). Later, colonies were established at Northern Illinois University (O.A. Schjeide), the University of Illinois (F.A. Kummerow), the University of Wisconsin-Madison (W.H. McGibbon and J.J. Bitgood), and Purdue University (RGE). At present, only two R/O colonies exist world-wide: one at The Pennsylvania State University (RGE) and the other at the Medical University of Vienna, Austria (WJS).
2. Reproductive characteristics—hemizygous R/O females
2.1. Ovarian and oviduct morphology and yolk structure
Ovaries of R/O females tend to resemble those undergoing retrogression in WT hens (Hutt et al., 1956), and the follicles never attain mature size (i.e., they do not proceed from F4 to F1 in the follicular hierarchy). Thus, instead of containing approximately 6–10 hierarchal (vitellogenic) follicles in a graduated succession of sizes (8–37 mm) as described by Johnson (2000), R/O ovaries typically contain approximately 15 follicles with a maximal diameter of around 20 mm (equal to follicles typically classified as F4 or F5; Fig. 1). In addition, follicles varying in both color (from pale yellow to green to chocolate brown) and shape (some contain multiple nodules and are thus raspberry-like in appearance) are evident (Ho et al., 1974; Jones et al., 1975; Grau et al., 1979; Mitchell et al., 1979; Nimpf et al., 1989; Elkin et al., 2003b; Ocón-Grove et al., 2007; Giles et al., 2010). As compared to WT layers, R/O hens have smaller oviducts with less active mucous glands (Ho et al., 1974). The latter characteristic was attributed to long-term disuse and was not considered to be the cause of non-laying (Ho et al., 1974). The lack of other oviductal abnormalities or of gross pathologic changes in R/O hens was confirmed by Mitchell et al. (1979).
Fig. 1.
Representative ovaries and blood plasma from sexually mature wild-type (WT) and ‘restricted ovulator’ (R/O) White Leghorn hens. In addition to lacking a typical ovarian follicular hierarchy and possessing abnormal follicles of varying shapes and colors, R/O blood plasma is extremely lipemic due to a marked accumulation of yolk precursor macromolecules in the circulation.
Within hierarchal follicles that are actively taking up yolk precursor macromolecules from the bloodstream, yolk deposition within the growing oocyte normally occurs in concentric pseudo-spherical shells that do not mix (Burley and Vadehra, 1989). This can be easily visualized either by feeding fat-soluble dyes to hens or by injecting the dye as a colloidal dispersion into their blood stream, hard-cooking (or freezing) the eggs, and then preparing a cross-section of the yolk. Using this method, Birrenkott and McGibbon (1975) and Grau et al. (1979) investigated the yolk ring structure of laid eggs from WT and R/O hens. Based on the dye deposition pattern, Birrenkott and McGibbon (1975) reported that yolks from WT and R/O hens took approximately 8 d and 7 d, respectively, to develop, but they did not mention whether there was any disruption of the normal yolk banding pattern in eggs from R/O hens compared with those from WT hens. In contrast, Grau et al. (1979) observed rings in the yolk of hierarchal follicles of R/O hens but, in laid R/O eggs, the yolk ring structure was completely disrupted. This suggested that the yolk structure of R/O eggs was not as stable as that of eggs from WT hens. Although the underlying cause of this phenomenon is unknown, Grau et al. (1979) found that yolks of R/O eggs contained 16% more water than WT egg yolks, while Schjeide et al. (1975) observed a paucity of maturing yolk granules in the ooplasm of small hierarchal follicles (5 mm in diameter) of R/O hens.
2.2. Egg production and yolk protein profiles
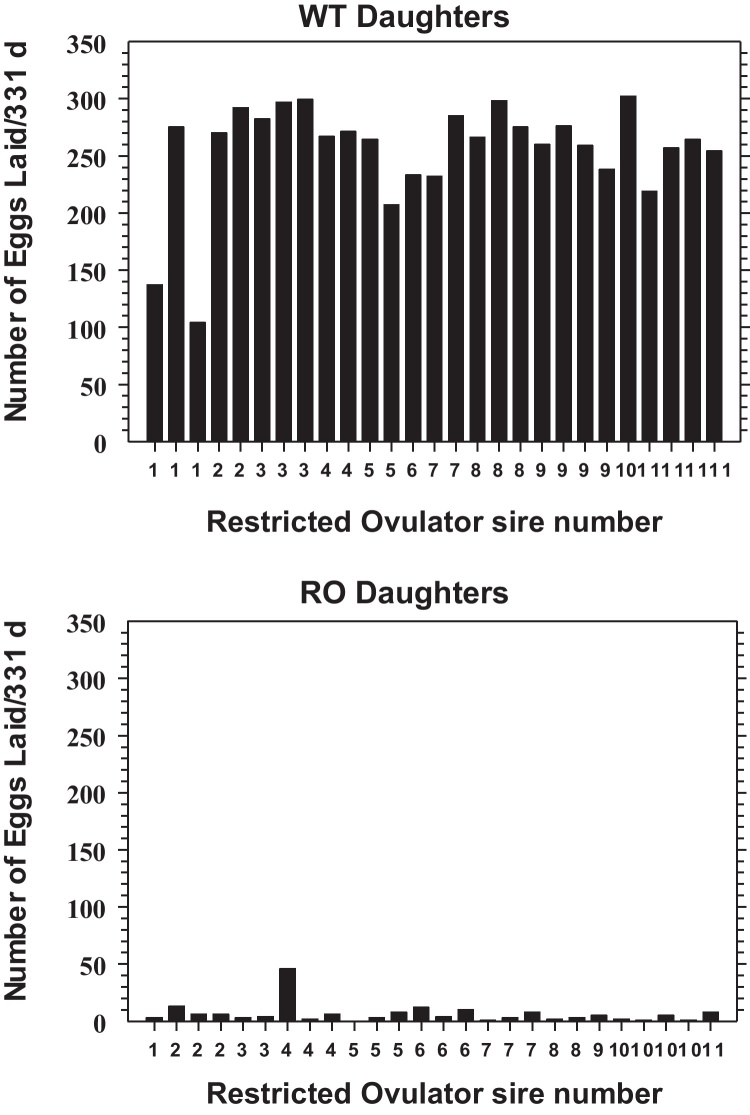
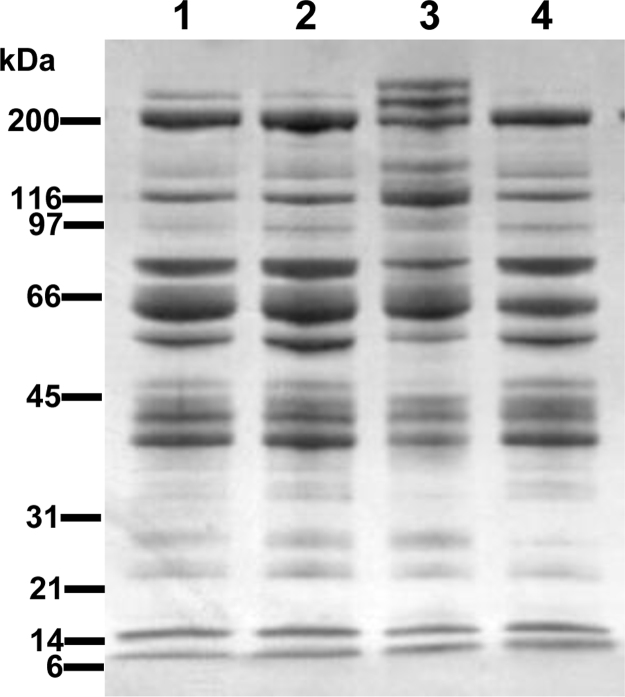
Despite the extreme changes in ovarian morphology, some R/O females are able to lay eggs (Table 1 and Fig. 2), although the eggs are generally smaller and have darker colored yolks than those from the normal hens (Ho et al., 1974; Jones et al., 1975; Birrenkott and McGibbon, 1975; Schjeide et al., 1976; Mitchell et al., 1979; Grau et al., 1979; Cho, 1981; Michel et al., 1984; Smith and Kummerow, 1988; Elkin et al., 2003b; Giles et al., 2010). Elkin et al. (2003b) reported that the SDS-PAGE profiles of yolk proteins from two eggs each from a WT hen and an R/O hen that laid 46 eggs in 331 d were very similar in terms of banding pattern (Fig. 3). However, in one of the R/O eggs, the cathepsin D-mediated proteolysis of the major yolk protein precursors, namely apolipoprotein B and vitellogenin (VTG) (Burley and Vadehra, 1989; Retzek et al., 1992), appeared to be somewhat incomplete. Overall, a change in the yolk protein profile per se is probably not responsible for the unstable yolk structure of laid R/O eggs described above.
Table 1.
Relative comparison of risk factors for ovarian cancer (OC) in women and the domestic hen (WT = wild-type; R/O = restricted ovulator).
| Item | Womena | WT Hensb | R/O Hensb |
|---|---|---|---|
| Incidence of OC | 1–2% | 27% | 3% |
| Lifetime ovulations | 400–500c | 422 Eggs | 28 Eggs |
| Progesterone | Protective | Normald | Lowere |
| Estrogen | Increases risk | Normald | Highere |
| Gonadotropins | Increases risk | Normald | Highere |
Data from Riman et al. (1998).
Data from Giles et al. (2010).
Approximate number.
Plasma levels of hormones.
Plasma levels of hormones relative to WT hens.
Adapted from Giles et al. (2010); reproduced with permission from Int. J. Gynecol. Cancer 20:738–744. Copyright IGCS and ESGO (2010).
Fig. 2.
Egg production of the daughters of mutant ‘restricted ovulator’ (R/O) sires. Individual egg production was monitored for either four or five PCR-genotyped daughters from each of 11 PCR-genotyped mutant (heterozygous) R/O sires for 331 d. During this period, the 27 wild-type (WT) daughters laid an average (±SD) of 255 ± 45 eggs, while the 26 R/O daughters averaged 6 ± 9 eggs laid.
From Elkin et al. (2003b); reproduced with permission from Poultry Science 82:517–525. Copyright Poultry Science Association, Inc. (2003).
Fig. 3.
SDS-PAGE (4.5–18% gradient) gel of whole yolk extracts from a wild-type (WT) and a ‘restricted ovulator’ (R/O) hen stained with Coomassie Brilliant Blue R-250. Protein standard molecular weights are indicated. Lanes 1 and 2 contained yolk extracts of two eggs from a WT hen; lanes 3 and 4 contained yolk extracts of two eggs from an R/O hen that laid 46 eggs/331 d (a daughter of sire 4 in Fig. 2). Each lane contained 12 μg of protein.
From Elkin et al. (2003b); reproduced with permission from Poultry Science 82:517–525. Copyright Poultry Science Association, Inc. (2003).
2.3. Reproductive hormones and receptors
Birrenkott (1975) hypothesized that the R/O phenotype was caused by an aberration in the hypothalamus or pituitary. Although he observed a normal response in circulating LH levels following the injection of R/O hens with GnRH, this did not rule out a problem at the hypothalamus. Plasma FSH levels were not determined. Birrenkott et al. (1975) also reported that plasma LH levels were significantly higher in R/O females as compared with their WT siblings, while progesterone values were similar between the two groups. Although many of the WT hens had 17β-estradiol concentrations that were below the sensitivity limit of the assay (5 pg/mL), the majority of the R/O hen plasma samples fell within the range of the standard curve. Birrenkott et al. (1975) suggested that the altered rates of hormone production or degradation might be responsible for the suppressed ovulation in R/O hens. However, subsequent molecular studies (discussed in Section 4) did not support this hypothesis.
In chickens, the basal estrogen (estradiol + estrone):progesterone ratio is a good predictor of ovulatory frequency (Leszczynski et al., 1984). Schjeide et al. (1976) reported an approximate 3-fold elevation in circulating estradiol and estrone in serum from R/O hens compared with that from normal layers (19 ng/100 mL and 25 ng/100 mL vs. 8 ng/100 mL and 8 ng/100 mL, respectively). They also mentioned that (unpublished) work at the University of Wisconsin had been unsuccessful in restoring normal ovarian function in R/O hens with hormone supplementation. Toda et al. (1983) confirmed the findings of Schjeide et al. (1976) with regard to 3-fold higher plasma estrogen levels in R/O hens compared with WT hens (approximately 30 ng/100 mL vs. 10 ng/100 mL, respectively), and also reported that the former had approximately 3-fold lower progesterone concentrations than WT hens (40 ng/100 mL vs. 140 ng/100 mL, respectively). Leszczynski et al. (1984) observed that R/O hens had a plasma estrogen:progesterone ratio that was 12.6 times higher than normal and resulted from 3-fold higher levels of estrogen and 4-fold lower levels of progesterone, as compared with WT layers. Moreover, R/O females did not exhibit any cycle-related variation in circulating progesterone levels and had smaller primary sex organs. This is in keeping with the observations that in chickens, almost all of the progesterone is secreted by only the most mature preovulatory (hierarchal) follicle (Johnson, 2000) and that the follicles of R/O hens typically never attain a size greater than F4 or F5 (Fig. 1).
Ocón-Grove et al. (2007) reported that, as compared with WT hens, R/O hens had an increased abundance of pituitary progesterone receptor (PR) mRNA and PR isoforms A and B (the two major PR variants expressed in the chicken), significantly elevated levels of plasma LH, FSH, estrone, and estradiol, and markedly attenuated levels of circulating progesterone. In mice, the PR has been shown to be essential for generating gonadotropin surges (Chappell et al., 1999). Ocón-Grove et al. (2007) hypothesized that the upregulation of PR mRNA and PR isoforms A and B was most likely a result of the marked hyperestrogenemia and hypoprogesteronemia, a hallmark of R/O hens. In addition, they suggested that the increased levels of circulating FSH in the mutant hens could have resulted from the lack of negative feedback inhibition from the ovary in the form of lower inhibin secretion. The lower plasma progesterone observed in R/O hens may have facilitated the secretion of GnRH (Ocón-Grove et al., 2007) or removed inhibition of a hypothalamic factor(s) responsible for increasing LH secretion from the pituitary gland.
2.4. Incidence of ovarian cancer
Because many cases are diagnosed at an advanced stage and the prognosis is poor, epithelial-derived ovarian cancer (OC) is the most fatal human gynecologic malignancy (Giles et al., 2010). In general, most animals do not spontaneously develop OC, although some avian species (including chickens) have a high incidence of ovarian neoplasms (Giles et al., 2010). The etiology of this malignancy is poorly understood, but one hypothesis proposes that an increased risk of OC is associated with increased ovulations (Fathalla, 1971), as the traumatized epithelium of ruptured follicles is repeatedly repaired and exposed to estrogen-rich follicular fluid (Riman et al., 1998).
The fact that R/O hens generally fail to ovulate may suggest that they would be less susceptible to developing OC if the “incessant ovulation” hypothesis of Fathalla (1971) holds true. However, R/O hens also exhibit a hormonal profile that is associated with an increased risk of developing OC (i.e., high circulating levels of estrogen and gonadotropins, with low levels of progesterone; Riman et al., 1998). Therefore, by taking advantage of a unique animal model (i.e., the R/O mutant hen), the relationship between OC and hormones in the absence of physical trauma to the ovarian surface epithelium (caused by ovulation) could be unambiguously assessed. In this regard, Giles et al. (2010) observed that only 1 of 31 R/O hens was diagnosed with OC as compared to 9 of 33 of their WT siblings (Table 1). This strongly suggested that in chickens, the number of ovulatory events is directly related to the incidence of OC. However, within the population of WT hens, evidence for a relationship between egg production and the incidence of OC was less obvious, although WT hens that developed OC laid a greater percentage of their eggs earlier in life (Giles et al., 2010).
3. Reproductive characteristics—heterozygous (‘carrier’) males
Based upon initial evaluations of roosters that carried the abnormal ro gene, Ho et al. (1974) determined that they were phenotypically normal in terms of reproductive ability, secondary sex characteristics, serum lipid concentrations, and reproductive tract anatomy. However, Ho et al. (1974) did not provide any supporting data, did not state upon what basis reproductive ability was assessed, and did not indicate how many roosters were evaluated. In an attempt to quantify the fertility and reproductive abilities of 11 heterozygous R/O carrier roosters and 18 of their WT siblings, Elkin and Zhong (2002) conducted a breeding trial. Individual semen samples were collected once weekly from the 29 males and used to artificially inseminate two hens each per rooster for 4 wk (roosters were 37–40 wk of age). The hens were from an unrelated line of White Leghorn chickens and had a documented rate of lay in excess of 80%. A total of 765 and 477 eggs were incubated from all matings of the WT and R/O sires, respectively, equating to approximately 21 eggs from each mating triad. There was no effect of rooster genotype on fertility, hatchability of fertile eggs, and hatchability of all eggs set, which averaged approximately 77%, 93%, and 71%, respectively. Although chicks from WT sire matings were, unexplainably, 1 g heavier than those from R/O matings (38 g vs. 37 g, respectively; P < 0.05), Elkin and Zhong (2002) considered the reproductive function of R/O sires to be normal, thus confirming the earlier qualitative statements of Ho et al. (1974).
4. Molecular characterization of the R/O mutation
Information on any of the proteins involved in oocyte growth and systemic lipoprotein transport in the laying hen was lacking until 1989, when it was demonstrated that the absence of a 95-kDa protein in R/O hens is responsible for the impaired uptake of VLDL into oocytes in-vivo and, consequently, for the failure of oocytes to develop normally (Nimpf et al., 1989). Stifani et al. (1990) subsequently showed that this single 95-kDa protein in the plasma membrane of the oocyte also served as a receptor for VTG, the other major yolk lipoprotein.
4.1. Identification of a point mutation in the VLDL/VTG receptor (LR8)
The ligand specificity, as well as the immunological cross-reactivity of the protein with antibodies against the human LDL receptor and the calcium requirement for ligand binding, predicted that it was a homologue of mammalian LDL receptors (LDLRs). The LDLR and relatives thereof (the group of receptors is designated LR, for LDLR relatives) comprises a large number of genes whose products contain a characteristic set of structural domains (for details, see Schneider and Nimpf, 2003). Attempts to delineate the evolutionary history of this gene family must take into consideration their wide range of functions, which include the binding to the extracellular domains of an ever growing list of ligands, ligand uptake through clathrin-mediated mechanisms, and signal transduction via the cytoplasmic regions of the receptors (Stolt and Bock, 2006). Knowledge gained in studies about the LR gene family in the chicken has provided major new insights into the biology of these receptors, both in structural and functional terms (Schneider et al., 1997).
Molecular cloning of the cDNA specifying the 95-kDa oocyte protein indeed revealed that it is an LR containing a multifunctional ligand binding domain (Bujo et al., 1994). In contrast to the ligand binding domain of the LDLR, which consists of 7 tandemly arranged so-called binding repeats, the chicken receptor's binding domain was found to contain 8 such repeats. In addition to VLDL and VTG (Stifani et al., 1990; Barber et al., 1991), the receptor was shown to bind riboflavin-binding protein complexed with VTG (Mac Lachlan et al., 1994), clusterin (apolipoprotein J; Mahon et al., 1999), and apolipoprotein E (apoE), a mammalian apolipoprotein with homology to VTG (Steyrer et al., 1990). The avian receptor was subsequently named LR8 (LR with 8 ligand binding repeats) in order to distinguish it from mammalian “VLDL receptors” that had been reported to bind VLDL (Takahashi et al., 1992). The gene for LR8 is designated Gallus gallus VLDLR (NCBI CAA56505) and is located on the Z chromosome (Bujo et al., 1994; Nanda et al., 2000). The R/O allele carries a point mutation (G to C substitution), which results in the replacement of Cysteine-682 with a serine in the extracellular domain of LR8. Interestingly, the first ever delineated mutation in the human LDLR gene causing familial hypercholesterolemia occurred exactly in the equivalent position. The disruption of a disulfide bond by the loss of the cysteine residue due to this mutation was shown to lead to misfolding of the protein accompanied by rapid intracellular degradation of the altered receptor molecules (Leitersdorf et al., 1990). As a consequence, LR8 does not reach the plasma membrane of oocytes of R/O hens, and is unable to mediate the uptake of serum-derived yolk precursor molecules.
Although it is quite surprising that oocytes of R/O hens enter the hierarchal stage of oocyte growth at all, there are at least two possible explanations for this occurrence. One is based on the facile access of the oocyte surface to the serum compartment, as (i) the endothelium of capillaries in the follicular wall displays extensive fenestration (Perry et al., 1978) and (ii) the surrounding cell layers and the basement membrane are permeable to macromolecular complexes such as lipoproteins, VTG, and others (Hummel et al., 2004, 2007). These properties are compatible with bulk (or “fluid phase”) uptake of yolk precursors, which are present at high concentrations in the serum. Thus, this process, which occurs at high rate due to the large surface of the oocyte, may be characteristic of the earlier phase of oocyte growth, i.e., up to the largest size of oocytes seen in R/O ovaries (Fig. 1). In this scenario, however, LR8 is envisioned to be essential for completion of oocyte growth. Receptor-mediated endocytosis is a process in which the concentration of receptor ligands in the target cell reaches levels that exceed by several-fold that in the extracellular fluid (Goldstein and Brown, 2009), whereas in bulk fluid uptake, there is a simple linear relationship. Importantly, again different from nonspecific bulk uptake, the receptor-mediated pathway allows the uptake of specific components that have high affinity for the receptors. Together, these features of yolk precursor uptake via LR8 may be critical for the maturation of oocytes. In any case, ovulation of oocytes that have not reached the final mature state can occur, possibly explaining the observations (Ho et al., 1974; Jones et al., 1975; Birrenkott and McGibbon, 1975; Schjeide et al., 1976; Mitchell et al., 1979; Grau et al., 1979; Cho, 1981; Michel et al., 1984; Smith and Kummerow, 1988; Elkin et al., 2003b; Giles et al., 2010, Table 1 and Fig. 2) that (i) R/O hens occasionally lay eggs, and (ii) these eggs are smaller. The second, alternative or additional, explanation for early phase R/O oocyte growth is that at least in the early stages of yolk uptake, endocytic receptor(s) other than LR8 may perform this task. One proposed candidate for such a role is a 380-kDa protein with many hallmark properties of yolk precursor binding proteins (Stifani et al., 1991; Bujo et al., 1997). Interestingly, as determined by quantitative PCR, the mRNA level for this protein termed LRP380, which is localized within, or closely associated with, the oocyte plasma membrane is 11-times higher in the ovarian stroma of R/O hens than of normal hens (T. Zarrabi, Diploma Thesis, University of Vienna, 2002; and D. Raich, unpublished). This increase may be a compensatory mechanism for the LR8 defect in R/O hens. However, although LRP380 contains sequences with high homology to signature domains typical of LRs (Stifani et al., 1991; Nimpf et al., 1994), it has not been shown directly to be competent for internalization of ligands, nor has its complete structure been delineated (WJS, personal communication). Therefore, further studies are needed to ascertain the physiological and biochemical roles of LRP380 in oocyte growth.
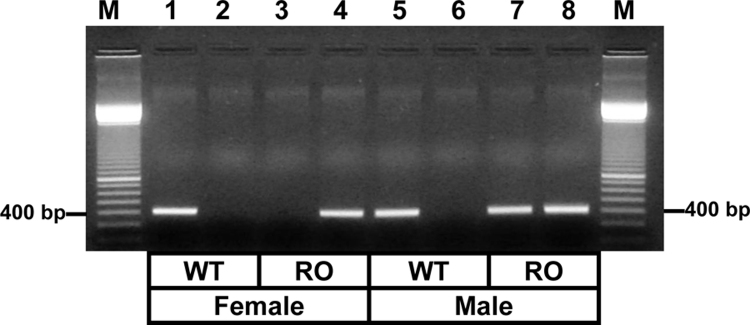
In addition to providing exciting molecular insights into the biology of R/O hens (see also Section 6), the delineation of the mutation facilitated the development of a rapid PCR-based genotyping procedure for the identification of mutant alleles in R/O animals (Fig. 4; Bujo et al., 1996; Elkin et al., 2003b). Needing only microliter amounts of blood (red cells, which in avians are nucleated), this rapid and accurate procedure eliminated the need for conventional progeny testing while reducing the time needed to identify R/O carrier males and mutant females from approximately one year to two days or less.
Fig. 4.
PCR amplification of genomic DNA from wild-type (WT) and ‘restricted ovulator’ (R/O) chickens. The PCR-amplified fragments obtained from 150 ng of genomic DNA isolated from erythrocytes were generated using primer pairs specific for the WT (Lanes 1, 3, 5, and 7) or the mutant very low density lipoprotein receptor genes (Lanes 2, 4, 6, and 8), respectively. The PCR-amplified products were subjected to agarose gel electrophoresis as described by Bujo et al. (1996). Lanes 1 and 2: WT female; Lanes 3 and 4: mutant R/O female; Lanes 5 and 6: WT male; and Lanes 7 and 8: mutant R/O carrier male. Lanes M contain as size markers the 100-base pair (bp) ladder.
From Elkin et al. (2003b); reproduced with permission from Poultry Science 82:517–525. Copyright Poultry Science Association, Inc. (2003).
The biological role of chicken LR8 in the laying hen is thus unambiguously documented by both biochemical and genetic evidence (Bujo et al., 1995b; Nimpf et al., 1989). As described above, LR8 mediates a key step in the reproductive effort of the hen, i.e., normal oocyte growth. With respect to R/O roosters (Section 3), Elkin and Zhong (2002) concluded that only one normal allele for the LR8 gene is sufficient for optimal male reproductive performance. Accordingly, they suggested that LR8 production from the WT allele is able to compensate for the failure of the mutant allele to produce a normal gene product (i.e., the male phenotype, if any, is inherited as a recessive trait).
Interestingly, those tissues which express the VLDLR in mammals (i.e., heart, skeletal muscle, brain, and adipose tissue, but not the liver), express LR8 in the chicken, albeit at very low levels (approx. 0.5%) as compared to the oocytes. While mammals and somatic cells of the chicken express mainly a longer splice variant of VLDLR/LR8, chicken oocytes apparently express only the shorter isoform (Bujo et al., 1995a).
From these results, the following conclusions can be drawn. Oocytic LR8 is a multifunctional receptor, which transports lipoproteins and other components required for embryonic growth into the yolk (Schneider et al., 1998). The larger variant produced by somatic cells likely performs similar functions in mammals and oviparous species, as they express this isoform in the same tissues. The physiological properties of LR8 strengthen the view (Steyrer et al., 1990) that the avian receptor is the product of an ancient gene that has retained the ability to interact with many, if not all, ligands of younger members of the LR family. In this context, VTG, absent from therians, and apoE, not found in birds, have certain common biochemical properties and regions of sequence similarities, and have been suggested to be functional analogues (Steyrer et al., 1990). Thus, triglyceride-rich particles could be transported into metabolically active tissues (such as muscle, where VLDLR is abundant), while in avian oocytes VTG and VLDL are taken up to provide nutrients and energy for the developing embryo.
5. Other metabolic or physiological characteristics of mutant R/O hens
5.1. Tissue and plasma lipids and lipogenic enzyme activities
When averaged across all studies reported to date that employed sexually mature birds aged 6 mo and older, R/O hens had approximately 4.5-, 4.4-, and 4.9-times greater circulating levels of total cholesterol, phospholipids, and triglycerides, respectively, as compared with WT hens, and approximately 6-, 18-, and 56-fold higher levels, respectively, as compared with WT roosters (Table 2). Cho (1981) determined the plasma fatty acid profiles of cholesteryl esters, phospholipids, and triglycerides in normal layers and in R/O hens as they aged from 17 to 34 wk of age. Palmitic acid was the predominant circulating saturated fatty acid, while oleic and linoleic acids were the main unsaturated fatty acids. However, as the hens aged, no consistent trends in individual fatty acid concentrations were observed across the three lipid classes or two genotypes (Cho, 1981).
Table 2.
Plasma total lipids of sexually mature (>6 mo) wild-type (WT) hens, restricted ovulator (R/O) hens, and WT roosters.a
| Lipids | Cholesterol | Phospholipids | Triglycerides | |
|---|---|---|---|---|
| (mg/100 mL) | ||||
| WT hens | Avg | 159 | 694 | 1513 |
| SD | 91 | 288 | 1045 | |
| Range | 84–439 | 540–1021 | 570–3792 | |
| R/O hens | Avg | 717 | 3041 | 7421 |
| SD | 211 | 1783 | 3674 | |
| Range | 455–1380 | 910–4800 | 1777–13840 | |
| WT roosters | Avg | 110 | 172 | 131 |
| SD | 26 | 15 | 65 | |
| Range | 79–157 | 160–189 | 39–229 | |
Data from Ho et al. (1974), Ho (1976), Schjeide et al. (1976), Mitchell et al. (1979), Toda et al. (1980, 1981), Tokuyasu et al. (1980), Cho (1981), Qureshi et al. (1983), Cho et al. (1984, 1987), Leszczynski et al. (1984), Smith et al. (1985, 1987), Smith and Kummerow (1988, 1989), Elkin et al. (2003b, 2006).
R/O hens also generally have greater body weights (BW) and larger livers, both on an absolute and relative BW basis, that contain more total cholesterol and crude fat, and less crude protein, than those of WT hens or WT roosters (Ho et al., 1974; Ho, 1976; Mitchell et al., 1979; Fitch et al., 1982; Cho, 1983; Qureshi et al., 1983; Elkin et al., 2006). Moreover, as compared with normal layers, R/O hens exhibit elevated hepatic contents of myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, arachidonic acid, and docosahexaenoic acid, on both a relative and a total organ basis (Elkin et al., 2006).
Qureshi et al. (1983) measured the hepatic, intestinal, and adipose tissue activities of two key enzymes of cholesterol metabolism (discussed in Section 5.2), as well as various lipogenic enzymes, including fatty acid synthase, glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, malic enzyme, and citrate cleavage enzyme. When expressed on a cytosolic mass basis, the activities of all of the lipogenic enzymes in each tissue were depressed in R/O hens as compared with normal layers. Qureshi et al. (1983) concluded that the liver is the primary site for lipogenesis and cholesterogenesis in the chicken. Cho (1983) also observed markedly reduced rates of lipogenesis in R/O hens as evidenced by very low levels of incorporation of radiolabeled acetate and mevalonate into lipids. In contrast to normal laying hens, neither compound was readily oxidized to CO2.
5.2. Cholesterol metabolism and VLDL turnover rates
In contrast to marked differences in circulating cholesterol levels in normal layers and R/O hens (Table 2), the total cholesterol content of heart and other tissues is generally similar between genotypes (Ho, 1976; Cho et al., 1984). Cho et al. (1984) therefore suggested that a large portion of endogenous cholesterol pool in R/O hens is confined mainly to the circulatory system, as opposed to being deposited in the tissues. A notable exception was the aorta, which is the main site of atherosclerotic lesions in the laying hen (discussed in Section 5.3).
Based on the results of a kinetic study of cholesterol metabolism, Ho et al. (1974) concluded that the hypercholesterolemia exhibited by R/O hens was not caused by the overproduction of cholesterol, but was mainly due to the failure to sufficiently remove cholesterol from the body via “excretion” in egg yolks. They also reported that, as compared with WT hens, R/O hens had attenuated rates of endogenous cholesterol synthesis and a much slower daily turnover of cholesterol. Nimpf et al. (1989) confirmed the latter finding by demonstrating that the plasma clearance of intravenously injected 125I-VLDL was markedly impaired in R/O hens compared with WT hens. They also observed attenuated levels of radioactivity in oocytes from R/O hens compared with those from WT hens. Based on ligand binding experiments with 125I-VLDL, Nimpf et al. (1989) also provided the first biochemical evidence that the receptor for VLDL was absent in R/O oocytes and suggested that this defect was responsible for the R/O phenotype (described in Section 4).
Ho et al. (1974) reported that, as compared with normal layers, R/O hens had a 6-fold increase in the size of the plasma cholesterol pool and a 55% reduction in the rate of total cholesterol synthesis. This led Mitchell et al. (1979) to examine the activities of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) and cholesterol 7α-hydroxylase, the rate-limiting enzymes for biosyntheses of cholesterol and bile acids, respectively. Mitchell et al. (1979) observed that hepatic HMGR activities in 26-wk-old R/O hens were only between 12% and 25% of those of immature (18-wk-old) hens, while the activity of this enzyme increased by more than 2-fold in 26-wk-old WT hens (Mitchell et al., 1979). Because the circulating cholesterol levels in nonlayers were five times greater than those of normal layers, Mitchell et al. (1979) attributed the low HMGR activities in R/O hens to the feedback inhibition of cholesterol biosynthesis. These investigators also found that after maturation, cholesterol 7α-hydroxylase activities were decreased in both WT and R/O hens, which was most likely due to the decreased availability of cholesterol substrate pools for bile acid synthesis (Mitchell et al., 1979). Fitch et al. (1982) and Qureshi et al. (1983) also observed lower HMGR and cholesterol 7α-hydroxylase activities in R/O hens compared with WT hens. Paradoxically, Cho et al. (1987) reported that R/O hens had a daily fecal bile acid excretion rate that was twice as great as in normal layers. They suggested that this was an adaptive change, albeit inadequate, of R/O hens to normalize plasma cholesterol concentrations in response to their extreme hyperlipidemia. Cho et al. (1987) also concluded that there was a lack of a relationship between egg laying capacity and the rate of daily fecal neutral steroid excretion, since the latter was similar in R/O and WT hens.
HMGR inhibitors, commonly referred to as statins, are widely used in human medicine to lower plasma LDL-cholesterol levels and have also been shown to be effective plasma and egg cholesterol-lowering agents when fed to normal laying hens (Elkin et al., 1999, 2003a). Since mutant R/O hens exhibit plasma cholesterol levels that are approximately 5-fold higher than WT hens (described in Section 5.1), Elkin et al. (2006) examined the effects of orally administered atorvastatin on plasma and liver cholesterol contents and the expression of several key genes regulating cholesterol and VLDL metabolism in R/O and WT hens. Atorvastatin lowered plasma cholesterol levels by approximately 60% in WT hens and by 45% in R/O hens; however, the statin treatment did not completely normalize the blood lipid levels of the R/O females. Expression of several key genes involved in VLDL assembly (such as apolipoprotein B, the large subunit of microsomal triglyceride transfer protein, and protein disulfide isomerase) were suppressed in R/O hens compared with WT hens, but were unaffected by atorvastatin treatment. In contrast, hepatic HMGR mRNA levels were elevated by atorvastatin in both WT and R/O hens, but only WT hens exhibited an atorvastatin-mediated increase in liver HMGR immunoreactive protein levels (Elkin et al., 2006). The reason why R/O hens responded less strongly than WT hens to atorvastatin treatment may lie in the fact that their hepatic HMGR gene expression and/or translation was down-regulated due to higher baseline levels of circulating cholesterol.
5.3. Atherosclerotic lesions and tissue oxidative stress
Chickens have been a valuable animal model for the study of atherosclerosis (Toda et al., 1983; St. Clair, 1998; Ayala et al., 2005). This condition develops naturally in their aortas and coronary arteries and at an accelerated rate when the birds are fed cholesterol-containing diets (St. Clair, 1998). Moreover, atherosclerotic lesions from cholesterol-fed chickens have cholesterol crystal inclusions, as evidenced by cholesterol clefts (spaces caused by the dissolving out of cholesterol crystals in sections of tissue embedded in paraffin; Stedman's, 2006), foam cells (monocyte-derived macrophages filled with modified lipids), and a fibromuscular cap, all of which are characteristics of human lesions (St. Clair, 1998).
Ho et al. (1974) were the first to report that R/O hens developed moderate to severe aortic atherosclerosis after 18 months of endogenous hyperlipidemia (i.e., when birds are approximately 2 years of age). Lesions typically contained a conglomeration of yellow plaques while, microscopically, thickened intima containing lipid-laden foam cells, together with large amounts of mucopolysaccharides, were observed. Ho et al. (1974) found no evidence of ulceration, calcification, or thrombosis of atherosclerotic plaques, although the aortas of R/O hens contained much greater amounts of cholesterol than those of WT hens or roosters. In a subsequent study, Ho (1976) confirmed this latter finding. Tokuyasu et al. (1980) examined aortas from 1- to 3-yr-old WT and R/O hens and found that lesions developed earlier and were more extensive and fatty in the R/O hens compared with the normal layers. However, stainable lipids in the aortic lesions increased with age in both groups. Moreover, Tokuyasu et al. (1980) observed that in R/O hens, the ascending thoracic and abdominal aorta were the most extensively and consistently diseased, with intimal lesions in the latter section containing foam cells, large pools of glycosaminoglycans, cholesterol clefts, extracellular lipid droplets up to 25 μm in diameter, and smooth muscle cells containing various amounts of lipids.
Toda et al. (1980) examined the coronary arteries of normal layers, R/O hens, and roosters and observed that most lesions were found in the proximal portion of both the left and right coronary arteries. The severity and speed of development of coronary atherosclerosis was highly correlated with the degree of hyperlipidemia. Ultrastructurally, the arterial lesions contained thickened intima with a plethora of foam cells and smooth muscle cells. Toda et al. (1981) subsequently conducted a light- and electron microscopic evaluation of aortas, coronary arteries, and superior mesenteric arteries from 3- to 7-yr-old roosters, WT hens, and R/O hens. Only mild pathological changes were noted in roosters as compared with layers and non-layers (R/O hens), with R/O hens exhibiting the most severe lipid-rich aortic lesions and the greatest degree of intimal thickening in the coronary and superior mesenteric arteries. Lesions in the latter two arteries were similar to those described by Toda et al. (1980).
Oxidized lipids, particularly oxidized LDL, are thought to initiate and modulate the inflammatory cellular events in the arterial wall and the formation of macrophage foam cells (Nosadini and Tonolo, 2011). Toda et al. (1983) observed large amounts of peroxidized lipids in both plasma and aortic tissue of R/O hens. Likewise, Smith et al. (1985) reported that, as compared with normal 1-yr-old laying hens, R/O hens exhibited extreme hyperlipidemia with increased levels of peroxidized plasma lipids. However, when expressed on the basis of triglyceride or phospholipid, there were no differences in the extent of plasma peroxidation. Smith and Kummerow (1988, 1989) supplemented the diets of WT and R/O hens with 1000 IU/kg diet of vitamin E, reported to function intercellularly and intracelluarly as an antioxidant (McDowell, 2000). Supplemental dietary vitamin E had no effect on the circulating levels of cholesterol or triglycerides in R/O hens, which were each approximately 10-fold higher than in WT hens. In contrast, vitamin E reduced plasma lipid peroxidation (determined by quantification of malondialdehyde) and aortic intimal thickness more than 4-fold in R/O hens, resulting in similar levels to those in WT hens. Smith and Kummerow (1988, 1989) concluded that serum lipid peroxidation was of greater importance than hyperlipidemia in the atherosclerotic process, since hyperlipidemic hens with normal serum peroxide levels showed no aortic intimal thickening. It should be pointed out that in man, despite strong biological and observational data, more rigorous scientific evaluation has not supported a causative relationship between dietary vitamin E and/or vitamin C supplements and a lowering of coronary artery disease risk (Tinkel et al., 2012).
5.4. Bone characteristics and egg yolk mineral composition
The laying hen skeleton contains cortical, trabecular, and medullary bone (Kim et al., 2004). The latter, which is located in the marrow cavity of most long bones (e.g., tibia and femur), is formed shortly before the onset of lay under the influence of estrogen and testosterone (Johnson, 2000). During the hen's 24–28-hr ovulation-oviposition cycle, medullary bone is resorbed and supplies approximately 35–40% of the daily calcium needs for eggshell formation (Comar and Driggers, 1949; Mueller et al., 1964); hens fed a high-calcium diet are generally able to restore medullary bone and calcium reserves when shell formation is not taking place (Johnson, 2000).
Since R/O hens typically lay few, if any, eggs in their lifetimes, Michel et al. (1984) took advantage of this characteristic in order to investigate whether physiological factors associated with egg production influenced the level of fluorine (F) deposition in the tibias of R/O and WT hens fed diets containing either 27 ppm F or 282 ppm F. They observed almost 2-fold greater tibial F contents in WT layers compared with R/O hens fed either diet, and 5-fold higher F levels due to dietary F supplementation for each genotype. Michel et al. (1984) suggested that once dietary F had been deposited into bones, it was less readily resorbed than calcium and became more concentrated over time, thus resulting in higher bone F levels in WT hens.
Kim et al. (2004) assessed the bones of WT, R/O and out-of-production (OP) hens; unlike non-laying R/O hens, the latter do not display secondary sex characteristics. As compared with WT and OP hens, R/O hens had higher circulating calcium concentrations, greater humerus, femur, and tibia ash contents, and higher bone mineral contents and densities as determined by dual-energy X-ray absorption. R/O females also exhibited extremely dense femur medullary bone deposition, as determined by gross and histological assessments. Kim et al. (2004) concluded that R/O hens have a higher bone density than WT or OP hens due to intensive medullary bone formation and the lack of a cyclic calcium metabolism as a consequence of grossly diminished egg shell formation.
Grau et al. (1979) examined the ring structure and relative iron content of yolks by freezing intact eggs from R/O and W/T hens, fixing them with formalin, cutting them in half and then staining one half with potassium dichromate and the other half with acidic potassium ferrocyanide. Yolks from R/O eggs stained less intensely with acidic ferrocyanide than WT yolks, suggesting that they contained relatively less iron. Mineral analyses performed by atomic absorption spectrophotometry revealed that R/O egg yolks contained less iron and copper, similar levels of phosphorus, and more sodium and potassium than egg yolks from WT hens (Grau et al., 1979). In addition, the yolk granule fraction of R/O eggs contained less iron and manganese than those from WT layers.
Iron in yolk is bound primarily to phosvitin, which is derived from circulating VTG following its intraoocytic processing by cathepsin D (Retzek et al., 1992). Grau et al. (1979) concluded that the ro gene may interfere with normal iron absorption, metabolism, or utilization leading to both hyperlipidemia and low-iron eggs. Since the hyperlipidemia in R/O hens results from a single gene defect not directly related to iron metabolism (described in Section 4), it is plausible that either proportionately less VTG and/or VTG carrying less iron, are delivered to the yolk of growing oocytes of R/O hens. However, the yolk protein profiles of eggs from WT and R/O hens analyzed by SDS-PAGE (Fig. 3) do not support the first possibility.
6. Studies in which the R/O strain has contributed to clarifying biological questions
6.1. The chicken LDL receptor
Molecular characterization of the chicken LDLR was achieved 9 years after that of LR8 (Hummel et al., 2003); the difficulties in obtaining full-length cDNA were due to its low levels of expression and the sluggish regulation by sterols in comparison to LDLRs in mammalian species. In contrast to LR8, which is expressed and functions essentially only in growing female germ cells, the chicken LDLR is ubiquitously expressed. Its main physiologic role in mammals is to supply somatic cells with cholesterol. However, in chickens, this function is less important in peripheral tissues, as these appear to satisfy their demand for cholesterol primarily via intracellular biosynthesis and, as a consequence, LDLR levels are low in e.g., cultured fibroblasts (Hummel et al., 2003). Accordingly, induction of LDLR levels in-vitro requires not only the absence of sterols from the culture medium, but also suppression of cellular cholesterol synthesis (see Section 5.2). Of particular interest in this context was the question of whether in steroidogenic tissues, cellular biosynthesis would suffice to supply the amounts of cholesterol required for conversion to steroids, or if a contribution by LDL taken up via the LDLR is required. Thus, the levels of LDLR protein were analyzed in adrenal glands and ovarian stroma of WT and R/O hens (Fig. 3 in Hummel et al., 2003). Interestingly, the LDLR levels were higher in the adrenals than in the ovary of animals of both genotypes, with the highest levels observed in the adrenals of WT hens. This indicated that: (i) chicken adrenal glands utilize cholesterol delivered via the LDLR cholesterol in addition to the intracellularly produced sterol; (ii) the elevated plasma cholesterol in R/O hens reduces the required contribution by the LDLR; and (iii) the hyperestrogenemia in R/O hens does not lead to induction of LDLR in steroidogenic tissues. However, estrogen administration to roosters increased hepatic LDLR levels significantly, and a similar difference was observed in the livers of untreated roosters versus mature WT hens (Hummel et al., 2003). Thus, the R/O hen provided additional insight into regulatory and physiological aspects of the LDLR in the chicken.
6.2. Endophilins in oocyte growth
The R/O hen has also been used as a tool in a study of important molecules, the endophilins, which are part of the accessory machinery of endocytic receptors such as LR8 (Hirayama et al., 2003). In general, endophilins have a prominent support function in processes that require remodeling of the membrane structure, such as the invagination of the plasma membrane during clathrin-mediated endocytosis (Kjaerulff et al., 2011). In the study of the three isoforms of endophilin (I-III) involving R/O hens, it was shown for the first time that endophilin III is the main isoform in oocytes, and endophilin II predominates in the cells of the follicular wall. Subsequently, the question was raised whether diminished yolk precursor uptake by R/O oocytes might be accompanied by an altered level of endophilin III. The results showed that the absence of LR8 function in R/O hens correlated with reduced levels of endophilin III in follicles harboring growing oocytes, but not in brain, compared to the levels in these tissues of WT hens (Hirayama et al., 2003). Thus, by utilizing the R/O hen in this study, it could be shown that oocytic endophilin III levels correlate with endocytic activity. However, upregulation of endophilin III does not appear to be a mechanism for compensation of receptor dysfunction in R/O hens.
6.3. Phospholipid transfer protein
In the circulation, phospholipid transfer protein (PLTP) transfers phospholipids from the surface of triacylglyceride-rich lipoproteins (such as VLDL) towards high density lipoprotein (HDL) particles during intravascular lipolysis (Albers et al., 2012). Chicken PLTP, first molecularly characterized in 2009 (Saarela et al., 2009), performs the function of remodeling HDL particles in a fashion reminiscent of its mammalian counterparts. However, given the significantly different lipoprotein classes in birds, in particular between mature roosters and hens, it was of interest to include the R/O hen as a further tool in a study of the functional properties of PLTP in the chicken. The PLTP activity in plasma of adult R/O hens was twice as high as that in mature and immature WT hens, adult WT roosters, and adult R/O roosters. Chicken PLTP is found primarily on HDL particles, which lack apoE as well as apoA-II, apolipoproteins that have been implicated in the distribution of PLTP among mammalian lipoprotein particles (Kärkkäinen et al., 2002). In this context, a second important function of HDL-associated PLTP is to increase the rate of cholesterol efflux from cells by HDL, the so-called reverse cholesterol transport pathway. This activity is determined by measuring the cholesterol released into the medium of cholesterol-loaded cells in the presence of the acceptor (Vikstedt et al., 2007). Interestingly, the most active cholesterol acceptor in this system was found to be HDL of mature R/O hens, which was twice as efficient as human HDL3, previously the best known acceptor. This physiologically beneficial property is possibly related to the comparatively low amount of free cholesterol in the R/O HDL particles, since it is not influenced by their triacylglycerol content (Saarela et al., 2009). Despite their dramatically hyperlipidemic status and propensity of R/O hens to develop lipid-rich aortic lesions (see Section 5.3), reports of death attributed to total arterial blockage(s) do not appear in the literature (see Section 5.3). This fact might possibly be explained by the high PLTP and efflux-stimulating activity of the plasma of R/O hens. Moreover, in the late 1990s, it was observed by RGE (unpublished) that there may be a subpopulation of R/O hens that die at a faster rate than their WT siblings within the first year of life, but that those hens which survive thereafter exhibit mortality rates that are similar to WT hens. Thus, it is tempting to speculate that such hens may be protected by higher endogenous PLTP activities than the ones that die during the first year. In summary, the experiments with R/O lipoproteins in investigations of chicken PLTP have facilitated insights of pathophysiological relevance that could not have been obtained in WT hens.
7. Relationship of the R/O phenotype to another model of compromised female fertility: The Watanabe Heritable Hyperlipidemic (WHHL) Rabbit
Due to a functional deficiency caused by a mutation in the LDLR gene, the WHHL rabbit is a widely used animal model for human autosomal dominant familial hypercholesterolemia, which is also caused by mutations in the LDL receptor gene, and atherosclerosis (Shiomi and Ito, 2009). However, its reproductive ability is markedly lower than that of normal rabbits (Shiomi et al., 1987; Mortensen and Frandsen, 1996) and abnormalities have been found in both sexes (Donnelly et al., 1991). For example, losses at ovulation, implantation, and during gestation have been reported in female WHHL rabbits, while males exhibited poor sperm morphology and motility (Donnelly et al., 1991). Robins et al. (1994) suggested that the LDL receptor defect in WHHL rabbits might contribute to their lower fecundity by attenuating steroidogenesis, as a result of reduced transport of cholesterol ester-rich LDL into steroid-producing cells. In concert with this hypothesis, WHHL rabbits exhibit decreased plasma estrogen levels (Robins et al., 1994). In addition, WHHL rabbits also had higher baseline plasma LH and FSH concentrations as compared with New Zealand White (NZW) rabbits (Robins et al., 1994), which putatively resulted from a lack of negative feedback inhibition from the ovary (Chappell et al., 1999). However, in contrast to the report of Donnelly et al. (1991), Robins et al. (1994) did not observe decreased concentrations of circulating progesterone at baseline or during the first 8 days of pseudopregnancy in WHHL rabbits, as compared to control NZW rabbits, and concluded that there was a compensatory source of cholesterol for progesterone steroidogenesis during the preovulatory period and in early pseudopregnancy.
Although WHHL female rabbits and mutant R/O hens each have defective LDL receptor supergene family members, elevated circulating gonadotropins, and are reproductively dysfunctional, they exhibit markedly divergent plasma ovarian steroid profiles. Therefore, the mechanisms underlying the etiology of the abnormal hypothalamic-pituitary-ovarian axes in these unique lipoprotein receptor-deficient animal models are likely not identical. Nevertheless, as our understanding of the molecular physiology and biochemistry of avian oocyte growth continues to expand, in part due to studies of the R/O model, new analogies may emerge between avian and mammalian systems, which ultimately could help to answer important questions in reproductive biology.
Acknowledgements
Original research in the authors’ laboratories was partially sponsored by grants from Progen Biotechnik and Parke-Davis Pharmaceutical Research, and a fellowship under the OECD Cooperative Research Program, Biological Resource Management for Sustainable Agricultural Systems to R.G.E., and grants from the Austrian Science Fund (FWF; Project No. P-20218-B11), the Jubilee Fund of the Austrian National Bank, Herzfelder Family Endowment, and GEN-AU (Genomics of Lipid Disorders – GOLD; Austrian Ministry of Education, Science, and Culture) to W.J.S. The authors are grateful to J. James Bitgood, University of Wisconsin-Madison, for providing tissue samples and fertile eggs that allowed the authors to conduct various studies and establish R/O colonies at their respective institutions.
Contributor Information
R.G. Elkin, Email: relkin@psu.edu.
W.J. Schneider, Email: wolfgang.schneider@meduniwien.ac.at.
References
- Albers J.J., Vuletic S., Cheung M.C. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim. Biophys. Acta. 2012;1821:345–357. doi: 10.1016/j.bbalip.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala I., García Perez B., Doménech G., Castells M.T., Valdés M. Use of the chicken as an experimental animal model in atherosclerosis. Avian Poult. Biol. Rev. 2005;16:151–159. [Google Scholar]
- Barber D.L., Sanders E.J., Aebersold R., Schneider W.J. The receptor for yolk lipoprotein deposition in the chicken oocyte. J. Biol. Chem. 1991;266:18761–18770. [PubMed] [Google Scholar]
- Birrenkott, G.P., 1975. Physiological characteristics associated with the restricted ovulator (RO) phenotype of chickens. M.S. Thesis. University of Wisconsin, Madison.
- Birrenkott G.P., McGibbon W.H. Yolk development in restricted ovulators and their normal sibs. Poultry Sci. 1975;54:1734. (abstr.) [Google Scholar]
- Birrenkott G.P., McGibbon W.H., Burke W.H., Wentworth B.C. A hormonal profile of the genetic restricted ovulator (RO) Poultry Sci. 1975;54:1735. (abstr.) [Google Scholar]
- Bujo H., Elkin R.G., Lindstedt K.A., Nimpf J., Bitgood J.J., Schneider W.J. A rapid, polymerase chain reaction-based procedure for identifying mutant restricted ovulator chickens. Poultry Sci. 1996;75:1113–1117. doi: 10.3382/ps.0751113. [DOI] [PubMed] [Google Scholar]
- Bujo H., Hermann M., Kaderli M.O., Jacobsen L., Sugawara S., Nimpf J., Yamamoto T., Schneider W.J. Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family. EMBO J. 1994;13:5165–5175. doi: 10.1002/j.1460-2075.1994.tb06847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujo H., Hermann M., Lindstedt K.A., Nimpf J., Schneider W.J. Low density lipoprotein receptor gene family members mediate yolk deposition. J. Nutr. 1997;127(5 Suppl.):801S–804S. doi: 10.1093/jn/127.5.801S. [DOI] [PubMed] [Google Scholar]
- Bujo H., Lindstedt K.A., Hermann M., Dalmau L.M., Nimpf J., Schneider W.J. Chicken oocytes and somatic cells express different splice variants of a multifunctional receptor. J. Biol. Chem. 1995;270:23546–23551. doi: 10.1074/jbc.270.40.23546. [DOI] [PubMed] [Google Scholar]
- Bujo H., Yamamoto T., Hayashi K., Hermann M., Nimpf J., Schneider W.J. Mutant oocytic low density lipoprotein receptor gene family member causes atherosclerosis and female sterility. Proc. Natl. Acad. Sci. USA. 1995;92:9905–9909. doi: 10.1073/pnas.92.21.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley R.W., Vadehra D.V. John Wiley & Sons; New York: 1989. The Avian Egg: Chemistry and Biology. [Google Scholar]
- Chappell P.E., Schneider J.S., Kim P., Xu M., Lydon J.P., O’Malley B.W., Levine J.E. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
- Cho B.H.S. Endogenous hyperlipidemia and plasma fatty acid profiles of normal and hereditary nonlaying chickens. Comp. Biochem. Physiol. 1981;68B:19–23. [Google Scholar]
- Cho B.H.S. Lipid composition and lipogenic activities in the livers of normo- and hereditary hyperlipidemic chickens. Comp. Biochem. Physiol. 1983;76B:331–334. doi: 10.1016/0305-0491(83)90078-0. [DOI] [PubMed] [Google Scholar]
- Cho B.H.S., Hassan A.S., Egwim P.O., Park J.R. Fecal steroid excretion in chickens with hereditary hyperlipidemia. Proc. Soc. Exp. Biol. Med. 1987;186:84–89. doi: 10.3181/00379727-186-42589. [DOI] [PubMed] [Google Scholar]
- Cho B.H.S., Lawson L.D., Toda T., Kummerow F.A. Oxidation of fatty acid by heart mitochondria of chickens with endogenous hyperlipidemia. Biochem. Med. 1984;31:347–351. doi: 10.1016/0006-2944(84)90090-5. [DOI] [PubMed] [Google Scholar]
- Comar C.L., Driggers J.C. Secretion of radioactive calcium in the hen's egg. Science. 1949;109:282. doi: 10.1126/science.109.2829.282. [DOI] [PubMed] [Google Scholar]
- Donnelly T.M., Kelsey S.F., Levine D.M., Parker T.S. Control of variance in experimental studies of hyperlipidemia using the WHHL rabbit. J. Lipid Res. 1991;32:1089–1098. [PubMed] [Google Scholar]
- Elkin R.G., Furumoto E.J., Thomas C.R. Assessment of egg nutrient compositional changes and residue in eggs, tissues, and excreta following the oral administration of atorvastatin to laying hens. J. Agric. Food Chem. 2003;51:3473–3481. doi: 10.1021/jf0212441. [DOI] [PubMed] [Google Scholar]
- Elkin R.G., Yan Z., Zhong Y., Donkin S.S., Buhman K.K., Story J.A., Turek J.J., Porter R.E., Jr., Anderson M., Homan R., Newton R.S. Select 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors vary in their ability to reduce egg yolk cholesterol levels in laying hens through alteration of hepatic cholesterol biosynthesis and plasma VLDL composition. J. Nutr. 1999;129:1010–1019. doi: 10.1093/jn/129.5.1010. [DOI] [PubMed] [Google Scholar]
- Elkin R.G., Zhong Y. Assessment of reproductive function in mutant restricted ovulator carrier roosters. Poultry Sci. 2002;81:1280–1282. doi: 10.1093/ps/81.9.1280. [DOI] [PubMed] [Google Scholar]
- Elkin R.G., Zhong Y., Donkin S.S., Hengstschläger-Ottnad E., Schneider W.J. Effects of atorvastatin on lipid metabolism in normolipidemic and hereditary hyperlipidemic, non-laying hens. Comp. Biochem. Physiol. 2006;143:319–329. doi: 10.1016/j.cbpb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Elkin R.G., Zhong Y., Porter R.E., Jr., Walzem R.L. Validation of a modified PCR-based method for identifying mutant restricted ovulator chickens: substantiation of genotypic classification by phenotypic traits. Poultry Sci. 2003;82:517–525. doi: 10.1093/ps/82.4.517. [DOI] [PubMed] [Google Scholar]
- Fathalla M.F. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- Fitch M.E., Elson C.E., Burger W.C., Qureshi A.A. Regulation of lipid metabolism in restricted ovulator chickens by dietary supplementation with high protein barley flour or culture filtrate of Trichoderma viride. Fed. Proc. 1982;41:543. (abstr.) [Google Scholar]
- Giles J.R., Elkin R.G., Trevino L.S., Urick M.E., Ramachandran R., Johnson P.A. The restricted ovulator chicken: a unique animal model for investigating the etiology of ovarian cancer. Int. J. Gynecol. Cancer. 2010;20:738–744. doi: 10.1111/igc.0b013e3181da2c49. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Brown M.S. History of discovery: The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau C.R., Roudybush T.E., McGibbon W.H. Mineral composition of yolk fractions and whole yolk from eggs of restricted ovulator hens. Poultry Sci. 1979;58:1143–1148. [Google Scholar]
- Hirayama S., Bajari T.M., Nimpf J., Schneider W.J. Receptor-mediated chicken oocyte growth: differential expression of endophilin isoforms in developing follicles. Biol. Reprod. 2003;68:1850–1860. doi: 10.1095/biolreprod.102.012427. [DOI] [PubMed] [Google Scholar]
- Ho K.-J. Cholesterol contents of various tissues of chickens with exogenous or endogenous hypercholesterolemia. Am. J. Clin. Nutr. 1976;29:187–191. doi: 10.1093/ajcn/29.2.187. [DOI] [PubMed] [Google Scholar]
- Ho K.-J., Lawrence W.D.L., Lewis A., Liu L.B., Taylor C.B. Hereditary hyperlipidemia in nonlaying chickens. Arch. Pathol. 1974;98:161–172. [PubMed] [Google Scholar]
- Hummel S., Christian S., Osanger A., Heid H., Nimpf J., Schneider W.J. Identification of a novel chondroitin-sulfated collagen in the membrane separating theca and granulosa cells in chicken ovarian follicles: the granulosa-theca cell interface is not a bona fide basement membrane. J. Biol. Chem. 2007;282:8011–8018. doi: 10.1074/jbc.M606029200. [DOI] [PubMed] [Google Scholar]
- Hummel S., Lynn E.G., Osanger A., Hirayama S., Nimpf J., Schneider W.J. Molecular characterization of the first avian LDL receptor: role in sterol metabolism of ovarian follicular cells. J. Lipid. Res. 2003;44:1633–1642. doi: 10.1194/jlr.M300014-JLR200. [DOI] [PubMed] [Google Scholar]
- Hummel S., Osanger A., Bajari T.M., Balasubramani M., Halfter W., Nimpf J., Schneider W.J. Extracellular matrices of the avian ovarian follicle. Molecular characterization of chicken perlecan. J. Biol. Chem. 2004;279:23486–23494. doi: 10.1074/jbc.M312694200. [DOI] [PubMed] [Google Scholar]
- Hutt F.B. The Ronald Press Company; New York: 1964. Animal Genetics. [Google Scholar]
- Hutt F.B., Goodwin K., Urban W.D. Investigations of nonlaying hens. Cornell Vet. 1956;46:257–273. [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Whittow C.G., editor. Sturkie's Avian Physiology. fifth ed. Academic Press; New York: 2000. pp. 569–596. [Google Scholar]
- Jones D.G., Briles W.E., Schjeide O.A. A mutation restricting ovulation in chickens. Poultry Sci. 1975;54:1780. (abstr.) [Google Scholar]
- Kärkkäinen M., Oka T., Olkkonen V.M., Metso J., Hattori H., Jauhiainen M., Ehnholm C. Isolation and partial characterization of the inactive and active forms of human plasma phospholipid transfer protein (PLTP) J. Biol. Chem. 2002;277:15413–15418. doi: 10.1074/jbc.M112247200. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Ford B.C., Mitchell A.D., Elkin R.G., Leach R.M. Comparative assessment of bone among wild-type, restricted ovulator and out-of-production hens. Br. Poultry Sci. 2004;45:463–470. doi: 10.1080/00071660412331286172. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O., Brodin L., Jung A. The structure and function of endophilin proteins. Cell Biochem. Biophys. 2011;60:137–154. doi: 10.1007/s12013-010-9137-5. [DOI] [PubMed] [Google Scholar]
- Leitersdorf E., Tobin E.J., Davignon J., Hobbs H.H. Common low-density lipoprotein receptor mutations in the French Canadian population. J. Clin. Invest. 1990;85:1014–1023. doi: 10.1172/JCI114531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynski D., Hagan R.C., Rowe S.E., Kummerow F.A. Plasma sex hormone and lipid patterns in normal and restricted-ovulator chicken hens. Gen. Comp. Endo. 1984;55:280–288. doi: 10.1016/0016-6480(84)90113-8. [DOI] [PubMed] [Google Scholar]
- Mac Lachlan I., Nimpf J., Schneider W.J. Avian riboflavin binding protein binds to lipoprotein receptors in association with vitellogenin. J. Biol. Chem. 1994;269:24127–24132. [PubMed] [Google Scholar]
- Mahon M.G., Lindstedt K.A., Hermann M., Nimpf J., Schneider W.J. Multiple involvement of clusterin in chicken ovarian follicle development. Binding to two oocyte-specific members of the low density lipoprotein receptor gene family. J. Biol. Chem. 1999;274:4036–4044. doi: 10.1074/jbc.274.7.4036. [DOI] [PubMed] [Google Scholar]
- McDowell L.R. 2nd ed. Iowa State University Press; Ames, IA: 2000. Vitamins in Animal and Human Nutrition. [Google Scholar]
- McGibbon W.H. Evidence that the restricted ovulator gene (ro) in the chicken is sex-linked. Genetics. 1977;86:s43–s44. [Google Scholar]
- Michel J.N., Suttie J.W., Sunde M.L. Fluorine deposition in bone as related to physiological state. Poultry Sci. 1984;63:1407–1411. doi: 10.3382/ps.0631407. [DOI] [PubMed] [Google Scholar]
- Mitchell A.D., Carlson S.E., McGibbon W.H., Goldfarb S. Hepatic HMG-CoA reductase and cholesterol 7α-hydroxylase activities in normal and hyperlipidemic-restricted ovulator atherosclerosis-prone chickens before and after the commencement of egg laying. Atherosclerosis. 1979;32:11–21. doi: 10.1016/0021-9150(79)90142-4. [DOI] [PubMed] [Google Scholar]
- Mortensen A., Frandsen H. Reproductive performance and changes in blood lipids in breeding females and in growing Watanabe heritable hyperlipidaemic and New Zealand White rabbits. Lab. Anim. 1996;30:252–259. doi: 10.1258/002367796780684854. [DOI] [PubMed] [Google Scholar]
- Mueller W.J., Schraer R., Schraer H. Calcium metabolism and skeletal dynamics of laying pullets. J. Nutr. 1964;84:20–26. doi: 10.1093/jn/84.1.20. [DOI] [PubMed] [Google Scholar]
- Nanda I., Zend-Ajusch E., Grützner F., Schartl M., Burt D.W., Koehler M., Fowler V.M., Goodwin G., Schneider W.J., Mizuno S., Dechant G., Haaf T., Schmid M. Conserved synteny between the chicken Z sex chromosome and human chromosome 9 includes the male regulatory gene DMRT1: a comparative (re)view on avian sex determination. Cytogenet. Cell Genet. 2000;89:67–78. doi: 10.1159/000015567. [DOI] [PubMed] [Google Scholar]
- Nimpf J., Radosavljevic M.J., Schneider W.J. Oocytes from the mutant restricted ovulator hen lack receptor for very low density lipoprotein. J. Biol. Chem. 1989;264:1393–1398. [PubMed] [Google Scholar]
- Nimpf J., Stifani S., Bilous P.T., Schneider W.J. The somatic cell-specific low density lipoprotein receptor-related protein of the chicken. Close kinship to mammalian low density lipoprotein receptor gene family members. J. Biol. Chem. 1994;269:212–219. [PubMed] [Google Scholar]
- Nosadini R., Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2011;21:79–85. doi: 10.1016/j.numecd.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Ocón-Grove O.M., Maddineni S., Hendricks G.L., III, Elkin R.G., Proudman J.A., Ramachandran R. Pituitary progesterone receptor expression and plasma gonadotropin concentrations in the reproductively dysfunctional mutant restricted ovulator chicken. Domest. Anim. Endocrinol. 2007;32:201–215. doi: 10.1016/j.domaniend.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Perry M.M., Gilbert A.B., Evans A.J. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J. Anat. 1978;125:481–487. [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.A., Abuirmeileh N., Fitch M., Elson C.E., Burger W.C., McGibbon W.H. Regulation of lipid metabolism in restricted ovulator chicken by dietary supplementation with HPBF and culture filtrate. Nutr. Rep. Int. 1983;27:87–95. [Google Scholar]
- Retzek H., Steyrer E., Sanders E.J., Nimpf J., Schneider W.J. Molecular cloning and functional characterization of chicken cathepsin D, a key enzyme for yolk formation. DNA Cell Biol. 1992;11:661–672. doi: 10.1089/dna.1992.11.661. [DOI] [PubMed] [Google Scholar]
- Riman T., Persson I., Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin. Endocrinol. 1998;49:695–707. doi: 10.1046/j.1365-2265.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- Robins E.D., Nelson L.M., Hoeg J.M. Aberrant hypothalamic-pituitary-ovarian axis in the Watanabe heritable hyperlipidemic rabbit. J. Lipid Res. 1994;35:52–59. [PubMed] [Google Scholar]
- Saarela J., Metso J., Schneider W.J., Jauhiainen M. Avian phospholipid transfer protein causes HDL conversion without affecting cholesterol efflux from macrophages. Biochim. Biophys. Acta-Mol. Cell. Biol. Lipids. 2009;1791:781–789. doi: 10.1016/j.bbalip.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Schjeide O.A., Briles W.E., Holshouser S., Jones D.G. Effect of “restricted ovulator” gene on uptake of yolk-precursor protein. Cell Tiss. Res. 1976;166:109–116. doi: 10.1007/BF00215130. [DOI] [PubMed] [Google Scholar]
- Schjeide O.A., Kancheva L., Hanzley L., Briles W.E. Production and fates of unique organelles (transosomes) in ovarian follicles of Gallus domesticus under various conditions. II. Cell Tiss. Res. 1975;163:63–79. doi: 10.1007/BF00218591. [DOI] [PubMed] [Google Scholar]
- Schneider W.J., Nimpf J. LDL receptor relatives at the crossroad of endocytosis and signaling. Cell. Mol. Life Sci. 2003;60:892–903. doi: 10.1007/s00018-003-2183-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W.J., Nimpf J., Bujo H. Novel members of the low density lipoprotein receptor superfamily and their potential roles in lipid metabolism. Curr. Opin. Lipidol. 1997;8:315–319. doi: 10.1097/00041433-199710000-00011. [DOI] [PubMed] [Google Scholar]
- Schneider W.J., Osanger A., Waclawek M., Nimpf J. Oocyte growth in the chicken: receptors and more. Biol. Chem. 1998;379:965–971. [PubMed] [Google Scholar]
- Shiomi M., Ito T. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of development: a tribute to the late Dr Yoshio Watanabe. Atherosclerosis. 2009;207:1–7. doi: 10.1016/j.atherosclerosis.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Shiomi M., Ito T., Watanabe Y. Effects of hyperlipidemia on the nursing ability of WHHL rabbits. Lab. Anim. Sci. 1987;37:84–88. [PubMed] [Google Scholar]
- Smith T.L., Kummerow F.A. Plasma lipid peroxidation and atherosclerosis in restricted ovulator chickens. In: Simic M.G., Taylor K.A., Ward J.F., von Sonntag C., editors. Oxygen Radicals in Biology and Medicine. Plenum Press; New York: 1988. pp. 941–944. [DOI] [PubMed] [Google Scholar]
- Smith T.L., Kummerow F.A. Effect of dietary vitamin E on plasma lipids and atherogenesis in restricted ovulator chickens. Atherosclerosis. 1989;75:105–109. doi: 10.1016/0021-9150(89)90166-4. [DOI] [PubMed] [Google Scholar]
- Smith T., Toda T., Kummerow F.A. Plasma lipid peroxidation in hyperlipidemic chickens. Atherosclerosis. 1985;57:119–122. doi: 10.1016/0021-9150(85)90143-1. [DOI] [PubMed] [Google Scholar]
- Smith T., Toda T., Toda Y., Kummerow F. The effect of elastase on chickens with endogenous hyperlipidemia. Biochem. Med. Metabol. Biol. 1987;37:96–100. doi: 10.1016/0885-4505(87)90014-4. [DOI] [PubMed] [Google Scholar]
- St. Clair R.W. The contribution of avian models to our understanding of atherosclerosis and their promise for the future. Lab. Anim. Sci. 1998;48:565–568. [PubMed] [Google Scholar]
- Stedman's . 28th ed. Lippincott, Williams & Wilkins; New York: 2006. Stedman's Medical Dictionary. [Google Scholar]
- Steyrer E., Barber D.L., Schneider W.J. Evolution of lipoprotein receptors. The chicken oocyte receptor for very low density lipoprotein and vitellogenin binds the mammalian ligand apolipoprotein E. J. Biol. Chem. 1990;265:19575–19581. [PubMed] [Google Scholar]
- Stifani S., Barber D.L., Aebersold R., Steyrer E., Shen X., Nimpf J., Schneider W.J. The laying hen expresses two different low density lipoprotein receptor-related proteins. J. Biol. Chem. 1991;266:19079–19087. [PubMed] [Google Scholar]
- Stifani S., Barber D.L., Nimpf J., Schneider W.J. A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin. Proc. Natl. Acad. Sci. USA. 1990;87:1955–1959. doi: 10.1073/pnas.87.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt P.C., Bock H.H. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18:1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Kawarabayasi Y., Nakai T., Sakai J., Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc. Natl. Acad. Sci. USA. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkel J., Hassanain H., Khouri S.J. Cardiovascular antioxidant therapy: a review of supplements, pharmacotherapies, and mechanisms. Cardiol. Rev. 2012;20:77–83. doi: 10.1097/CRD.0b013e31823dbbad. [DOI] [PubMed] [Google Scholar]
- Toda T., Leszczynski D., McGibbon W.H., Kummerow F.A. Coronary arterial lesions in sexually mature non-layers, layers, and roosters. Virchows Arch. A Path. Anat. Histol. 1980;388:123–135. doi: 10.1007/BF00430682. [DOI] [PubMed] [Google Scholar]
- Toda T., Leszczynski D., Nishimori I., Kummerow F. Arterial lesions in restricted-ovulator chickens with endogenous hyperlipidemia. Avian Dis. 1981;25:162–178. [PubMed] [Google Scholar]
- Toda T., Nishimori I., Kummerow F. Animal model of atherosclerosis. Experimental atherosclerosis in the chicken animal model. J. Japanese Atherosclerosis Soc. 1983;11:755–760. [Google Scholar]
- Tokuyasu K., Imai H., Taura S., Cho B.H.S., Kummerow F.A. Aortic lesions in nonlaying hens with endogenous hyperlipidemia. Arch. Pathol. Lab. Med. 1980;104:41–45. [PubMed] [Google Scholar]
- Vikstedt R., Metso J., Hakala J., Olkkonen V.M., Ehnholm C., Jauhiainen M. Cholesterol efflux from macrophage foam cells is enhanced by active phospholipid transfer protein through generation of two types of acceptor particles. Biochemistry. 2007;46:11979–11986. doi: 10.1021/bi700833h. [DOI] [PubMed] [Google Scholar]