The teenage years are often associated with ‘impulsive' behavior; that is, behavior with diminished regard to potential negative consequences. Adolescent impulsivity, while often adaptive, can manifest itself in a range of sub-optimal behaviors, including use of nicotine, alcohol, or illicit substances, symptoms associated with attention deficit hyperactivity disorder (ADHD), or poorer performance on laboratory assays of impulse control. Although these maladaptive behaviors are often co-morbid, their correlation is not perfect. It is therefore increasingly recognized that impulsivity is multi-dimensional, with some predicting that ‘what is generally denoted as impulsivity will be fractionated into distinct forms that may, however, often coexist in the same individual' (Dalley et al, 2011, page 691).
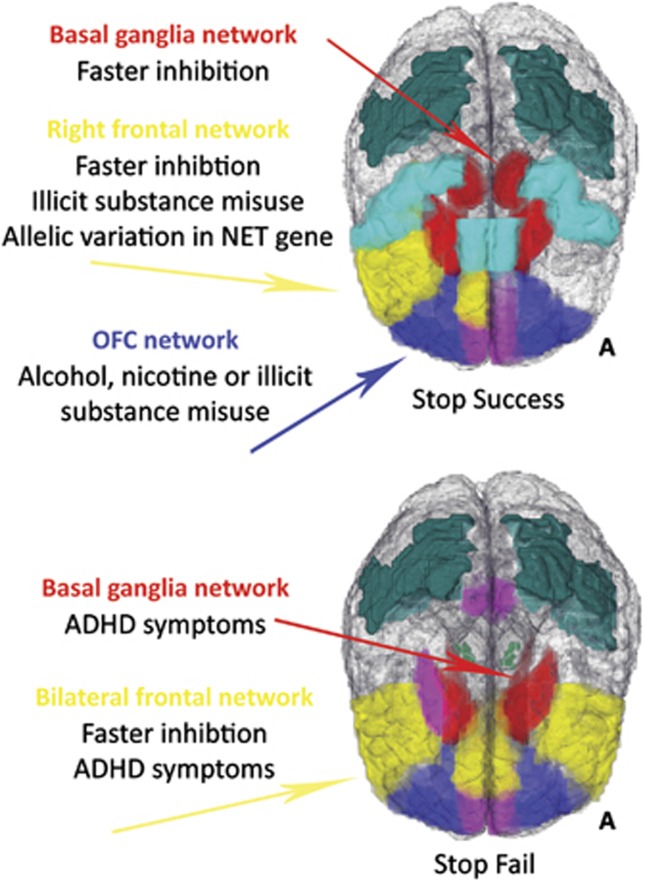
Fractionating impulsivity is challenging, not least because of the large sample size needed to ensure an adequate number of participants in each phenotypic group, although recently the ‘population neuroscience' (Paus, 2010) approach has provided these large samples. Data from the IMAGEN (Schumann et al, 2010) project permitted the data-driven identification of impulsivity subtypes by Whelan et al (2012). Nearly 1900 14-year-olds completed a test of motor inhibition—the Stop Signal Task (SST)—while undergoing functional magnetic resonance imaging (fMRI). The large sample size allowed functional brain activity to be decomposed into a smaller number of distinct networks using factor analysis (a data-reduction method). Next, these networks were tested for relationships with various phenotypes. Adolescents who had experimented with either alcohol, cigarettes, or illicit substances showed reduced activity in an orbitofrontal cortex network on successful stop trials, even those with only 1–4 total lifetime alcohol uses. For adolescents who had used illicit substances, there was hyperactivity in a right frontal network (inferior frontal gyrus, cingulate, and insula), an effect that remained even after controlling for nicotine and alcohol effects. In contrast, ADHD symptoms were associated with bilateral frontal (inferior frontal gyri, anterior cingulate, and anterior insula) and basal ganglia networks only on unsuccessful stop trials. Individual differences in the speed of the inhibition process on the SST were associated with activity in the right frontal network and in the basal ganglia. Finally, the right frontal network was also associated with allelic variation in a single-nucleotide polymorphism located in the SLC6A2 gene, which codes for the norepinephrine transporter (see Figure 1).
Figure 1.
The impulsivity networks and associated phenotypes described in Whelan et al (2012), for both trials on which subjects successfully inhibited an already initiated motor response (Stop Success) and trials on which subjects failed to inhibit (Stop Fail). A, anterior; ADHD, attention deficit hyperactivity disorder; OFC, orbitofrontal cortex; NET, norepinephrine transporter.
Understanding the neural correlates of impulsivity subtypes is important because it yields insights into the etiology of maladaptive impulsive behaviors. Disentangling the biological basis of substance misuse and ADHD symptoms has proven difficult previously because, for example, adult substance misusers are more likely to retrospectively endorse childhood ADHD symptoms (Ivanov et al, 2008). However, the results of Whelan et al (2012) suggest that ADHD symptoms and adolescent substance misuse can be separated, at least in terms of brain activity during a test of inhibitory control. Furthermore, these results support the role of norepinephrine in modulating impulse control, with implications for treatment of ADHD (Chamberlain et al, 2007). A goal of future research will be to shed more light on the structural, functional, neurochemical, and genetic underpinnings of the various impulsivity brain networks.
The authors declare no conflict of interest.
References
- Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Sch, ulz KP, London ED, Newcorn JH. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am J Drug Alcohol Abuse. 2008;34:239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- Paus T. Population neuroscience: why and how. Hum Brain Mapping. 2010;31:891–903. doi: 10.1002/hbm.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. The IMAGEN study: reinforcement-related behavior in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker G, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]