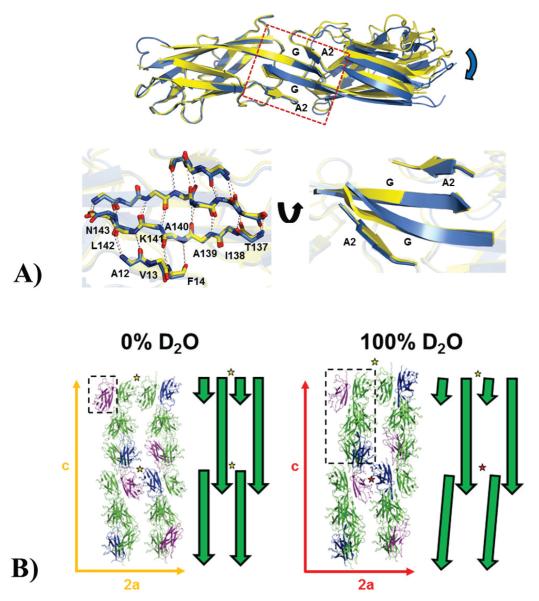
Figure 3.
(A) Effects of deuterium on the inter-domain domain-swapped dimer interface. Top panel: A SefDdscA dimer from a 100% D2O crystal (blue) is shown superimposed onto a single chain of a 0% D2O dimer (yellow) and clearly demonstrates a twisting and displacement of the other subunit of the dimer in the different solvents (blue arrow). The dimer interface which orchestrates these changes is boxed and the secondary structure is labeled. Bottom panel: (left) the box has been blown up and backbone residues in the A2 and G strands are shown as sticks with inter-sheet hydrogen bonds depicted as dashed lines. (Right) This region has been rotated to highlight the distortion in the sheet structure (B) Effect of deuterium on the overall packing in crystals of SefDdscA. Packing of SefDdscA in both crystal forms are shown along the a and c unit cell axes with the E (blue) and F (purple) chains in the 100% D2O crystal and equivalent positions in the 0% D2O crystal highlighted. The asymmetric units are depicted as dashed boxes. To the right of each is a schematic representation shown as green arrows. In the 0% D2O crystals, two ‘face-to-face’ dimers are shown (yellow star), however in the 100% D2O crystal, due to changes within the domain-swapped dimer interface (Fig. 2; Fig. 3; Supplementary Fig. 5; Supplementary Fig. 7) which are propagated down the c-axis, only one of these dimers is seen (yellow star), whilst to maintain lattice integrity, chains E and F do not form the usual dimer (red star).