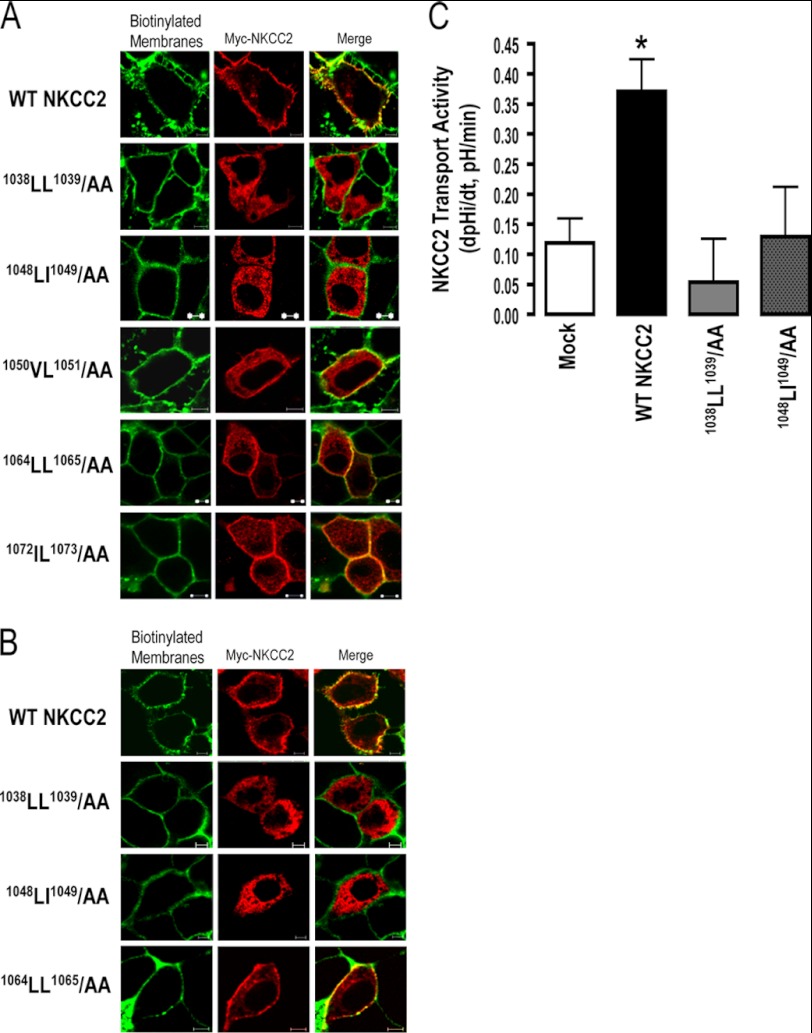
FIGURE 2.
1038AA1039 and 1048AA1049 proteins are not expressed at the cell surface. Immunolocalization studies of wild-type and mutated NKCC2 proteins. Cells were transiently transfected with Myc-tagged wild-type and mutant NKCC2 proteins, as indicated. Forty-eight hours post-transfection, membrane proteins of confluent OKP cells (A) or HEK cells (B) were biotinylated at 4 °C with the biotinylation reagent sulfo-NHS-SS-biotin. Then the monolayers were fixed and stained for Myc tag NKCC2 protein (Texas red) and cell surface biotin (avidin-Cy2). The stained specimens were evaluated by confocal microscopy. Optical sections (xy) at the cell surface are depicted for Texas red (red) or Cy2 channel (green), and a merged channel. The yellow color (merged image) represents co-localization of NKCC2 with biotinylated membranes, and therefore indicates correct targeting to the cell surface. The white bars represent 5 μm. C, measurement of Na-K-2Cl co-transport activity in OKP cells expressing WT NKCC2 or mutant proteins; 1038LL1039/AA and 1048LI1049/AA. Mean initial rate of pHi recovery (dpHi/dt) from NH4+-induced alkaline load. Each bar represents the mean ± S.E. rates of cell pH recovery (dpHi/dt, pH units/min) under different experimental conditions (mock cells, cells expressing Myc-NKCC2, cells transfected with Myc-1038LL1039/AA and cells transfected with 1048LI1049/AA). *, p < 0.05 versus WT NKCC2 (Mock, n = 7; WT NKCC2, n = 6; 1038LL1039/AA, n = 8 and 1048LI1049/AA, n = 7).