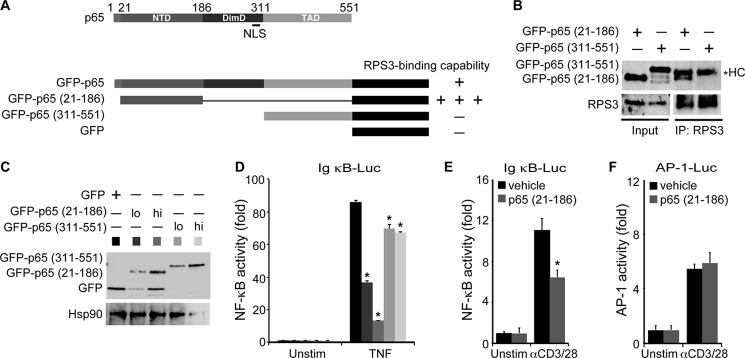
FIGURE 1.
p65 (21–186) fragment inhibits NF-κB gene expression. A, diagram of human p65 and the EGFP-fused full-length and indicated truncation mutants of p65. NTD, N-terminal domain; DimD, dimerization domain. The association capacities of the full-length and indicated truncated mutants of p65 to RPS3 are indicated as follows: −, no detectable interaction; +, detectable interaction; and +++, detectable strong interaction. B, whole cell lysates (Input) from HEK293T cells, transfected with either of the indicated EGFP-fused p65 truncated mutants, were immunoblotted directly or after immunoprecipitation (IP) with RPS3 antibody for the indicated proteins. The asterisk indicates nonspecific heavy chain (HC). C, HEK293T cells were transiently transfected with low dose (lo) or high dose (hi) of the 21–186 or 311–551 truncated mutants of p65 or EGFP vehicle control. After 24 h, the whole cell lysates were derived and immunoblotted directly with GFP antibody for the indicated EGFP fusion proteins. Hsp90 was used as a loading control. D, HEK293T cells were transfected as in C together with 5× κB-Luc reporter and pTKRL plasmids. After 24 h, the cells were left untreated (Unstim) or stimulated with TNF (50 ng/ml) and analyzed for luciferase. Shown are the means ± S.D. of relative NF-κB-driven luciferase activity (fold increase) (n = 3). *, p < 0.001 (Student's t test). E and F, Jurkat A3 cells were transiently transfected with EGFP vehicle control or p65 (21–186) truncated mutant, together with 5× κB-Luc reporter (E) or AP-1-Luc reporter (F) and pTKRL plasmids. After 48 h, the cells were left untreated (Unstim) or stimulated with CD3 and CD28 antibodies (1 μg/ml each, αCD3/28) and analyzed for luciferase. Shown are the means ± S.D. of relative NF-κB-driven (E) or AP-1-driven (F) luciferase activity (fold increase) (n = 3). *, p < 0.001 (E) and not significant (F) by Student's t test.