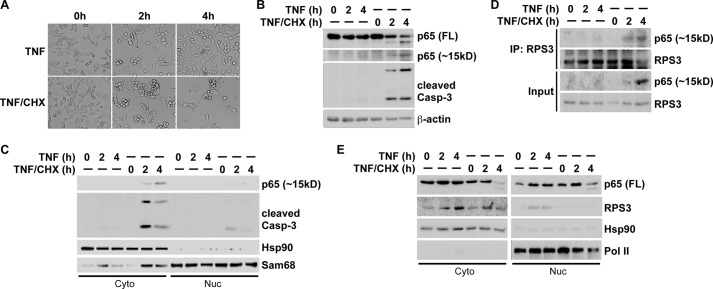
FIGURE 5.
Caspase 3-cleaved N-terminal 1–97 fragment of p65 interacts with RPS3 and attenuates NF-κB activation-induced RPS3 nuclear translocation in cells undergoing apoptosis. A, representative images of MEF cells, stimulated with TNF (50 ng/ml) alone or combined with cycloheximide (CHX, 10 μg/ml) for indicated period. B, whole cell lysates from MEF cells, stimulated as in A, were immunoblotted for full-length (FL), the C-terminal fragment, and the smaller N-terminal fragment (∼15 kDa) of p65 with the antibody specifically recognizing the N terminus of p65, and cleaved caspase 3, with β-actin as a loading control. C, immunoblot analysis of cytosolic (Cyto) and nuclear (Nuc) subcellular fractions of MEF cells, stimulated as in A, for cleaved N-terminal p65 and cleaved caspase-3. Hsp90 and Sam68 serve as cytosolic and nuclear markers, respectively. D, cytosolic subcellular fractions (Input) from MEF cells, stimulated as indicated, were immunoblotted directly or after IP with RPS3 antibody for RPS3 and the p65 N-terminal fragment (∼15 kDa). E, immunoblot analysis of cytosolic (Cyto) and nuclear (Nuc) subcellular fractions of MEF cells, stimulated as indicated, for full-length (FL) p65 and RPS3. Hsp90 and RNA polymerase II (pol II) serve as cytosolic and nuclear markers and loading controls, respectively.