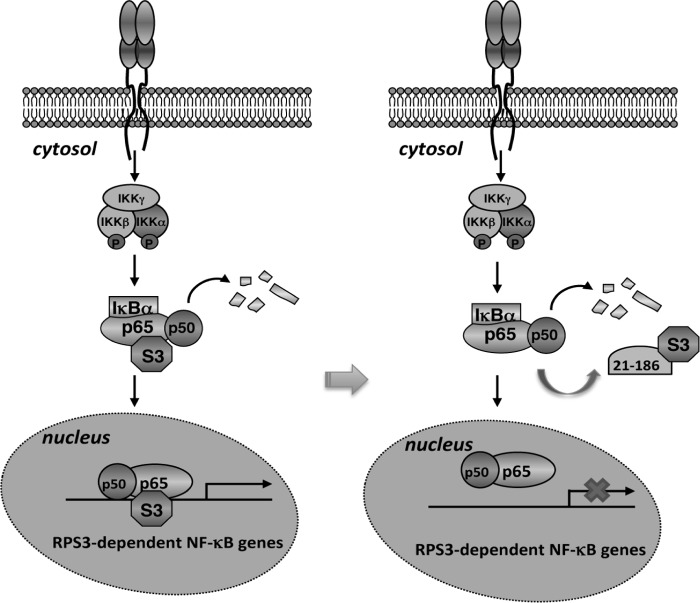
FIGURE 6.
Schematic model of selective attenuation of NF-κB gene transcription by the 21–186 truncated mutant of p65. Left panel, RPS3 exists in the cytoplasmic p65-p50-IκBα inhibitory complex in resting cells. NF-κB activation stimuli trigger the phosphorylation and degradation of IκBα, allowing p65, p50, and RPS3 to translocate to the nucleus for the transcription of a subset of NF-κB target genes. Right panel, because of its high affinity to RPS3, the 21–186 truncated mutant of p65 competes RPS3 off the p65-p50-IκBα inhibitory complex in the cytoplasm. Upon NF-κB activation, the 21–186 truncated mutant of p65 sequesters RPS3 in the cytoplasm but leaves p65 nuclear translocation to occur normally. Lacking RPS3, the binding of the NF-κB complex to the RPS3-dependent NF-κB target genes is diminished. Thus, the 21–186 truncated mutant of p65 selectively inhibits NF-κB gene transcription by targeting RPS3.