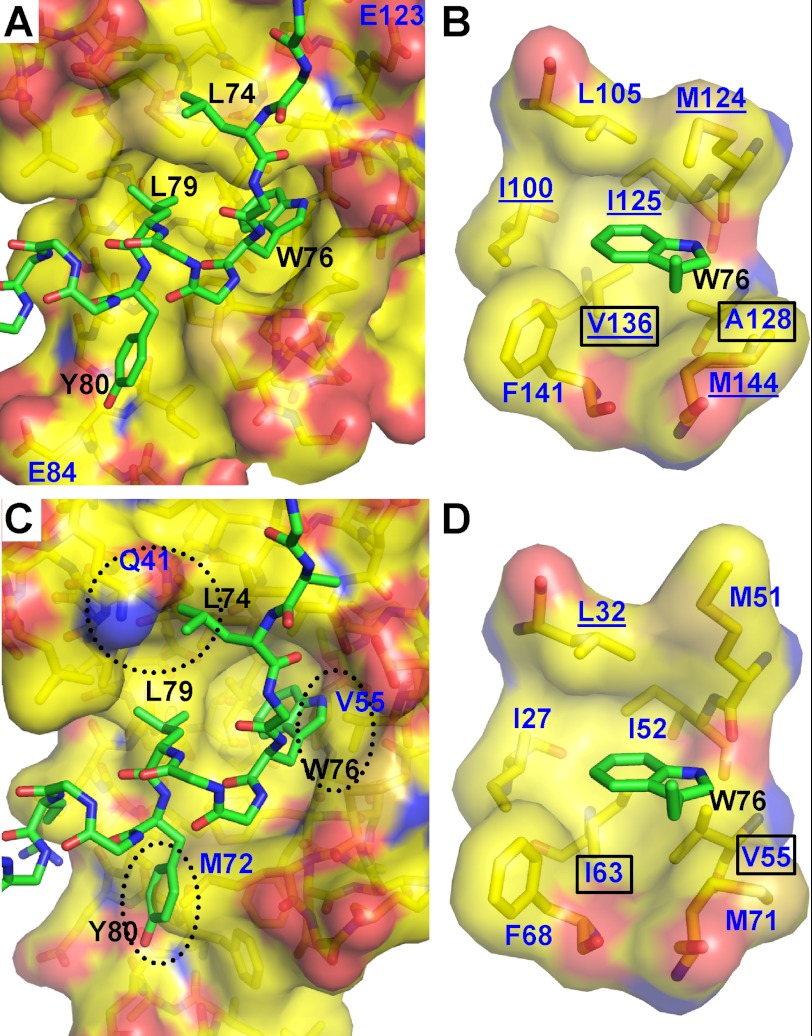
FIGURE 2.
Interaction of Orai1-CMBD with CaM-C and CaM-N (hypothetical). A, interaction of Orai1-CMBD with CaM-C. Residues of Orai1-CMBD are labeled in black, and CaM in dark blue. Leu74, Trp76, and Leu79 of Orai1-CMBD make major contributions to the hydrophobic interaction between CaM and Orai1-CMBD. Labeled in dark blue are Glu123 and Glu84 of CaM that may have electrostatic repulsion for A73E and Y80E mutants of Orai1-CMBD, respectively. The color scheme of atoms is the same as described in the legend to Fig. 1B. B, zoomed-in view of the interaction between Trp76 of Orai1-CMBD (green) and the hydrophobic pocket of calmodulin C-terminal domain (yellow). The hydrophobic pocket is shown as a surface representation. The residues composing the hydrophobic pocket of calmodulin and the side chain of Trp76 are shown as sticks. Residues of Orai1-CMBD are labeled in black, and CaM in dark blue. The color scheme of atoms is the same as described in the legend to Fig. 1B. C, hypothetical interaction of Orai1-CMBD with CaM-N. Structure of CaM-N was superimposed onto the CaM-C in complex with Orai1-CMBD, to mimic the interaction of CaM-N with Orai1-CMBD. This simple superposition introduces three major clashes between CaM-N and Orai1-CMBD, as indicated by the dotted oval. D, zoomed-in view of the hypothetical interaction between Trp76 of Orai1-CMBD (green) and the hydrophobic pocket of the calmodulin N-terminal domain (yellow). The scheme of the representation is the same as B. Two residues of the hydrophobic pocket that are different between CaM-N and CaM-C are boxed. The CaM residues that show NOE signals with Trp76 of Orai1-CMBD are underlined in B and D.