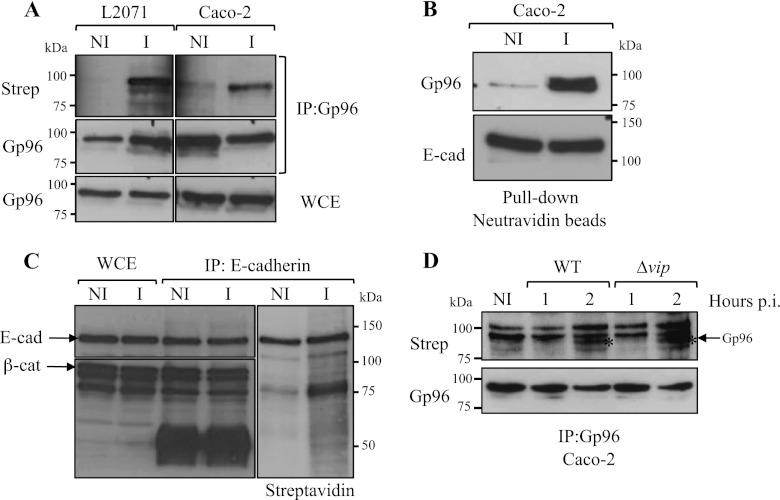
FIGURE 2.
L. monocytogenes infection induces the cellular redistribution of Gp96 and its exposure to the extracellular milieu. A, L2071 and Caco-2 cells were infected (I) with L. monocytogenes WT or left uninfected (NI). Surface-exposed proteins of infected and non-infected cells were biotinylated, and cells were lysed. Gp96 was immunoprecipitated (IP:Gp96) from total cell lysates, and immunoprecipitates were revealed for Gp96 and streptavidin. As a control, Gp96 was revealed in whole-cell extracts (WCE). B, Caco-2 cells were infected (I) with L. monocytogenes WT or left uninfected (NI). Surface-exposed proteins of infected and non-infected cells were biotinylated. Total cell extracts were subjected to a pulldown assay using neutravidin beads. Biotinylated proteins recovered by the neutravidin beads were revealed for Gp96 and as a control for E-cadherin (E-cad), a 120-kDa transmembrane protein. C, total extracts from surface biotin-labeled infected (I) or non-infected (NI) Caco-2 cells were immunoprecipitated with an anti-E-cadherin antibody. Immunoprecipitates were revealed for E-cadherin, β-catenin (β-cat; a 92-kDa intracellular protein), and streptavidin (Strep). D, surface localization of Gp96 induced by Listeria infection is Vip-independent. Cells were infected with L. monocytogenes WT or Δvip for 1 or 2 h. Surface proteins were labeled with biotin, and cells were lysed. Immunoprecipitates obtained with an anti-Gp96 antibody were revealed for Gp96 and streptavidin. The asterisk (*) indicates the band corresponding to Gp96. p.i., post-infection.