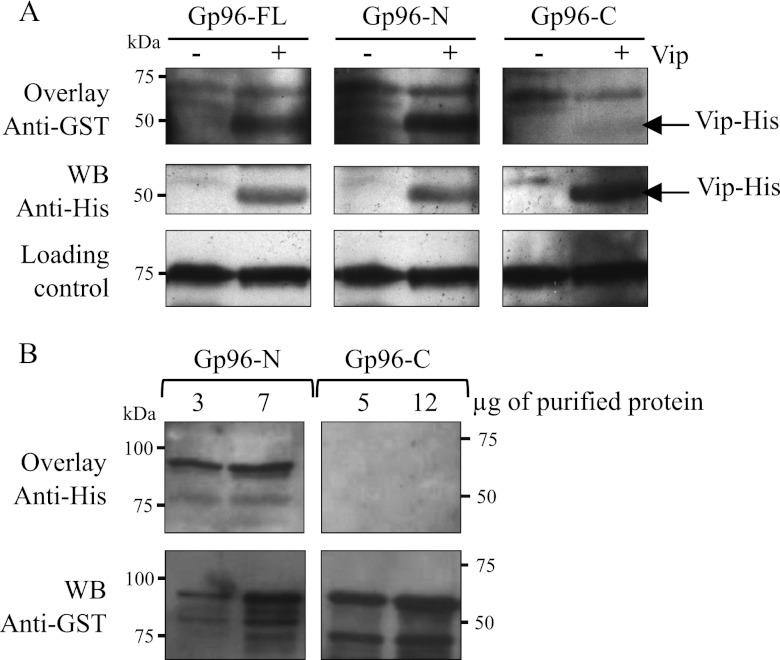
FIGURE 5.
The N-terminal part of Gp96 directly interacts with Vip in vitro. A, E. coli BL21(DE3) harboring a Vip-His tag expression vector were grown in the absence (−) or presence (+) of 0.1 mm isopropyl 1-thio-β-d-galactopyranoside to induce the expression of Vip-His. Total extracts of E. coli were separated on a polyacrylamide gel, transferred onto a membrane, and probed successively with 25 μg/ml purified GST-Gp96 variants (Gp96-FL, Gp96-N, or Gp96-C) and anti-GST (Overlay Anti-GST). As control, the same whole protein extracts were revealed with an anti-His antibody (WB Anti-His), showing the presence of Vip-His only in isopropyl 1-thio-β-d-galactopyranoside-induced (+) E. coli total extracts. Loading controls are shown for each condition. B, purified GST-Gp96-N and -C were separated on a polyacrylamide gel, transferred to a membrane, and probed successively with 25 μg/ml purified Vip-His and anti-His antibody (Overlay Vip-His). For the Gp96-N variant, 3 and 7 μg of purified protein were used. Higher amounts (5 and 12 μg) of Gp96-C were used. Western blot using anti-GST antibody was performed to control for the presence of GST-Gp96 proteins in the membrane (WB Anti-GST).