FIGURE 6.
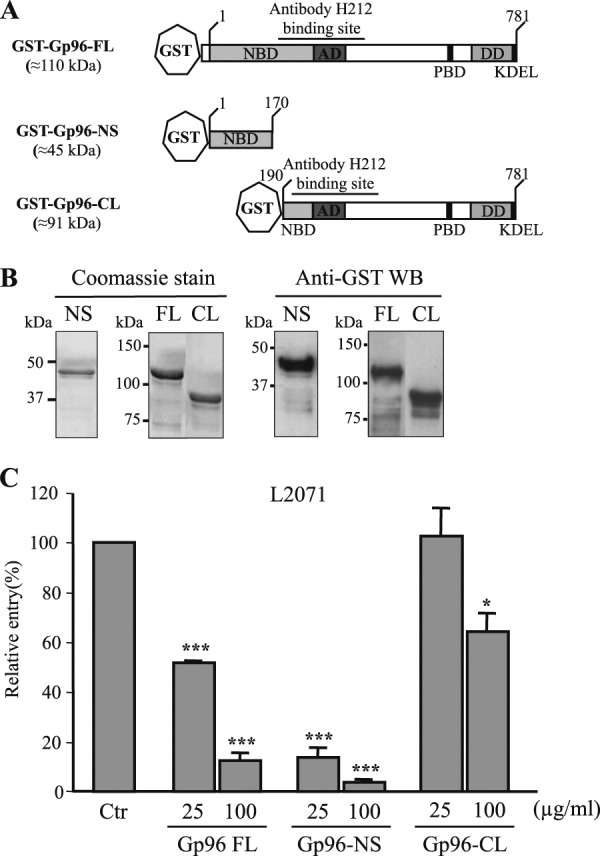
The N-terminal Gp96 (Asp1–Leu170) is sufficient to block Listeria invasion of L2071 cells. A, schematic representation of full-length and new truncated GST-Gp96 fusion proteins. Domains and amino acid residue positions are indicated. The N-terminal construct is shorter (GST-Gp96-NS). On the contrary, the C-terminal variant is longer (GST-Gp96-CL) and includes the H-212 antibody-binding site. B, Gp96-NS and -CL constructs were expressed in E. coli as GST fusion proteins and purified using a glutathione affinity chromatography approach. Purified truncated proteins (NS and CL) were analyzed by SDS-PAGE and Coomassie Blue staining. Western blots (WB) using anti-GST were also performed. Molecular masses are indicated. C, wild type L. monocytogenes were incubated with either 25 or 100 μg/ml purified GST-Gp96-FL, Gp96-NS, or Gp96-CL and used to infect L2071 cells. Entry levels of treated and NT bacteria were assessed by gentamicin survival assays. Values are given relative to the invasion of the non-treated bacteria into L2071 cells arbitrarily fixed to 100. Experiments were repeated three times in triplicate for each condition. Error bars represent S.D. Statistically significant differences are indicated: *, p < 0.05; ***, p < 0.001. NBD, nucleotide-binding site; AD, an acidic domain; PBD, peptide-binding domain; DD, region crucial for dimerization; Ctr, control.
