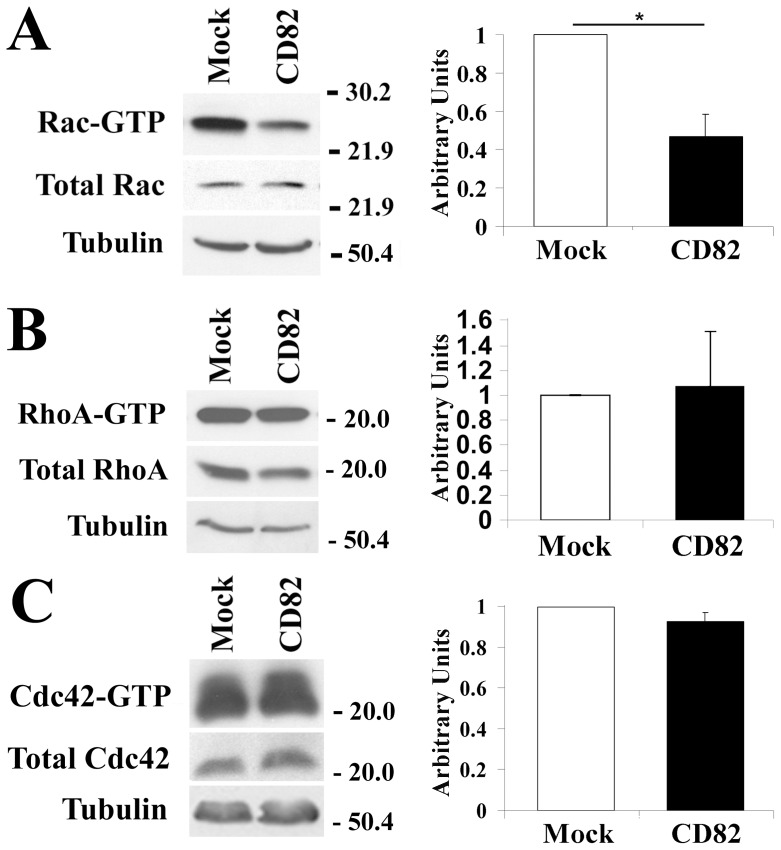
Figure 5. KAI1/CD82 regulates the activities of Rho GTPases.
( A ) KAI1/CD82 inhibits Rac1 activity. Du145-Mock or -KAI1/CD82 transfectant cells were lysed in a lysis buffer containing 1% NP-40 and 0.2% SDS detergents. Cell lysates were subjected to affinity precipitation with GST-PAK1, which binds to only the activated or GTP-bound Rac. The co-precipitated, GTP-bound Rac GTPase was detected by Rac mAb. The intact cell lysates were blotted with Rac mAb to demonstrate equivalent levels of total Rac proteins between Mock and KAI1/CD82 transfectant cells. Blots show the result from a representative experiment; the graph represents the relative density of the Rac band (mean±SD, n = 4), based on densitometric analysis. *: P<0.05. (B) KAI1/CD82 does not significantly alter RhoA activity. Du145 transfectants were pretreated as described above. GTP-bound RhoA was pulled down by GST-Rhotekin and detected by RhoA mAb. The blot shows the result from a representative experiment; the graph represents the relative density of the RhoA bands (mean±SD, n = 9), based on densitometric analysis. P>0.05 between Mock and KAI1/CD82. (C) KAI1/CD82 does not significantly alter Cdc42 activity. GTP-bound Cdc42 was pulled down by GST-PAK1 and detected by Cdc42 mAb. The blot shows the results from a representative experiment; the graph represents the relative density of the Cdc42 bands (mean±SD, n = 4), based on densitometric analysis. P>0.05 between Mock and KAI1/CD82. In all experiments, tubulin protein levels in cell lysates were detected via Western blot and served as protein loading controls.