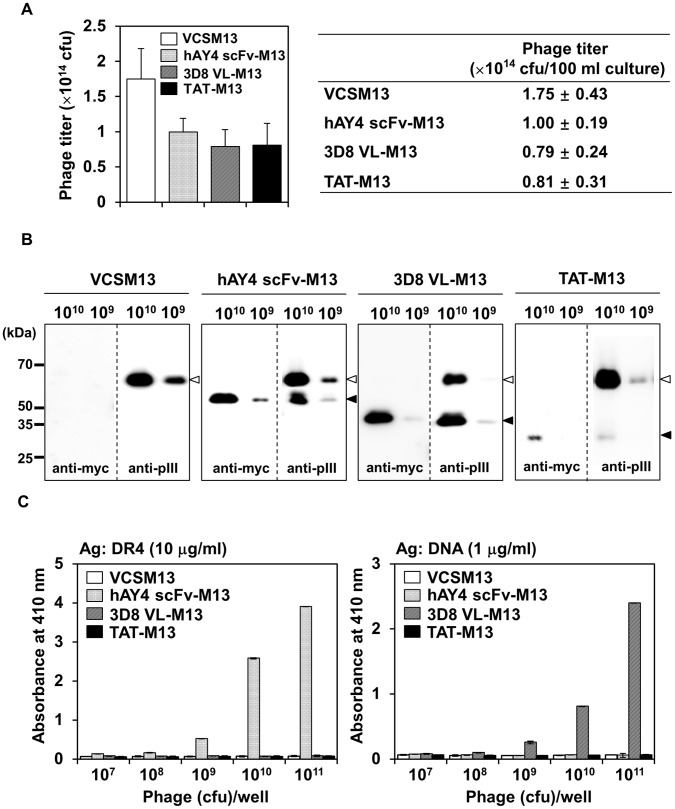
Figure 1. Generation of filamentous M13 phages displaying cell-penetrating 3D8 VL transbody (3D8 VL-M13) and TAT peptide (TAT-M13).
As a control, anti-DR4 hAY4 scFv without cell-penetrating ability was also employed; the phage particles are designated as hAY4 scFv-M13. (A) Phage titers obtained from 100-ml culture supernatants of recombinant phagemid-transformed bacteria by VCSM13 helper phage superinfection under optimal culture conditions as described in the text. Phage titers were determined by CFU assay. Data represent mean ± S.E. (error bars) of 5 independent experiments. (B) Western blot analysis of display efficiency of fusion proteins (insert-pIII) (filled arrow) versus full-length pIII (open arrow) from VCSM13 helper phage on the recombinant M13 filamentous phage particles. Equal titers (109 or 1010 CFU) of phage particles prepared as described in (A) were western blotted with anti-myc antibody to detect only pIII-fusion proteins (3D8 VL-pIII, and TAT-pIII, and hAY4 scFv-pIII) from the phagemid vectors or anti-M13 pIII antibody to detect both pIII-fusion proteins from the phagemid vectors and full-length pIII from the helper phage. The positions of molecular size marker are indicated. (C) Phage ELISA on DR4 or DNA to examine antigen-binding specificity of the recombinant phages. Various titers (107∼1011 CFU) of recombinant phages or VCSM13 helper phage were applied to each well, precoated with the indicated antigen. Bound phages were detected with HRP-conjugated anti-M13 antibody. Data represent mean ± S.E. (error bars) of three independent experiments carried out in triplicate.