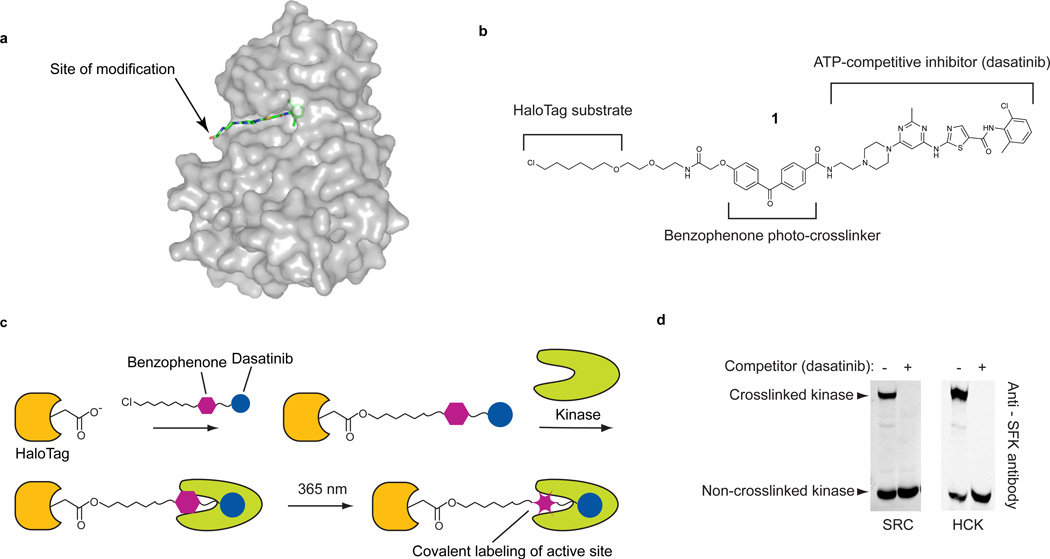
Figure 1. An active-site directed probe for ratiometric profiling of protein kinases.
(a) The cancer drug dasatinib in complex with the tyrosine kinase SRC (PDB code 3G5D). The arrow shows the site where dasatinib was modified with a benzophenone photo-crosslinker and an orthogonal chemical tag. (b) The chemical structure of probe 1. Probe 1 contains three components: (i) a potent ATP-competitive inhibitor (dasatanib), (ii) a photo-reactive benzophenone crosslinker, and (iii) a hexylchloride tag that selectively labels the active site of the self-labeling protein HaloTag. (c) Experimental crosslinking schematic using 1. Prior to photo-crosslinking experiments, HaloTag is labeled with 1. HT-1 is incubated with a kinase target and then irradiated with UV light. (d). HT-1 efficiently labels the recombinant SRC-family kinases (SFKs) SRC and HCK in cell lysate. Purified SRC or HCK (100 nM) was photo-crosslinked with HT-1 in mammalian cell lysate. Immunoblotting with an anti-SFK antibody shows that a large percentage of SRC and HCK are covalently modified. Upon addition of a dasatinib competitor, no mass-shifted kinases are observed.