Figure 4.
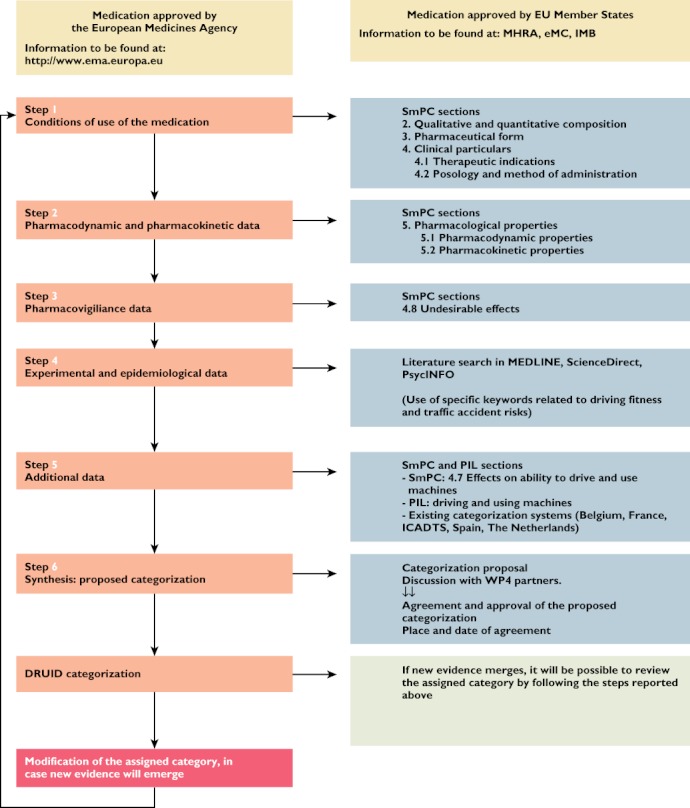
Flowchart representing the methodology that was followed during the DRUID categorization process. Legend: SmPC, Summary of Product Characteristics; PIL, Patient Information Leaflet; EMA, European Medicines Agency; MHRA, Medicines and Healthcare products Regulatory Agency; eMC, Electronic Medicines Compendium; IMB, Irish Medicines Board
