Abstract
Evidence has accumulated that the regulation of male sexual behavior by dopamine may not be the same in Japanese quail (and perhaps all birds) as it is in mammals. For example, the non-selective dopamine receptor agonist, apomorphine (APO) facilitates male sexual behavior in rats but inhibits it in quail. Although the general organization of the dopamine system is similar in birds and mammals, it is possible that the relative distribution and/or density of binding sites is different. We therefore compared the relative densities of D1-like and D2-like receptor subtypes in Japanese quail and rats, with the use of in vitro quantitative receptor autoradiography. Brain sections from 8 male rats and 8 male quail were labeled with [3H]SCH-23390 and [3H]Spiperone. In general we found a systematic species difference in the relative density of D1-like vs. D2-like receptors such that the D2/D1 ratio is higher in quail than in rats in areas well known to be important target sites for dopamine action such as striatal regions. We also uncovered significant differences in the relative density of D1-like and D2-like receptors in brain areas associated with sexual behavior, including the preoptic area, such that there was a greater D2/D1 ratio in quail as compared to rats. This difference may explain the variation in the behavioral effectiveness of APO in rats as compared to quail; with a higher relative density of D2-like receptors in quail, a similar dose of APO would be more likely to activate inhibitory processes in quail than in rats.
Keywords: dopamine, autoradiography, apomorphine, male sexual behavior, bird
INTRODUCTION
One of the most robust findings about dopamine, primarily based on mammalian studies, is that the stimulation of dopaminergic systems promotes the activation of male sexual behavior [Hull et al., 2002]. Compared to studies utilizing rats and other mammals (see [Hull et al., 2006] for a review), fewer studies have employed non-mammalian vertebrates such as Japanese quail (Coturnix japonica) to investigate the role of dopamine in the control of male sexual behavior, so we know relatively less about this relationship in quail [Balthazart and Ball, 1998]. Studies of the dopaminergic regulation of male sexual behavior in quail do indicate that it is important for the control of this behavior [Balthazart and Ball, 1998; Ball and Balthazart, 2002]. However, some data have been collected indicating that there may be significant species differences in the dopaminergic regulation of male behavior in quail as compared to rats.
Dopamine acts via two families of receptor sub-types, the “D1-like” and the “D2-like.” One primary way in which the signal-transduction mechanisms associated with these receptors varies is that by acting on the D1 receptors, dopamine stimulates adenylyl cyclase via a G protein to increase cAMP formation, whereas the activation of D2 receptors inhibits cAMP synthesis (reviewed in [Sibley and Monsma, 1992; Gingrich and Caron, 1993]). In rats there is substantial evidence indicating that the stimulation of both D1-like and the D2-like family of dopamine receptors is needed to facilitate the activation of male-typical copulatory behaviors [Hull et al., 2006]. For example, Scaletta and Hull (1990) showed that long-term castrated rats that no longer exhibit copulatory behavior can have this behavior partially restored after a systemic injection of apomorphine (APO), a non-selective dopamine receptor agonist, with or without testosterone. Furthermore, they found that microinjections of APO directly into the medial preoptic area (mPOA) of long-term castrated rats increased the number of observed mounts. They concluded that stimulation of some combination of D1/D2 dopamine receptors can partially restore copulation, and that dopamine receptors in the mPOA may contribute to sexual arousal in long-term castrates [Scaletta and Hull, 1990]. Subsequent studies indicated that D1 and D2 receptors in the mPOA specifically influence different aspects of copulatory behavior in male rats [Hull et al., 1992]. For example, a D1 agonist injected into the mPOA increased erections and decreased seminal emissions while a D1 antagonist and/or D2 agonist injected into the mPOA decreased erections and increased seminal emissions. In addition, a D2 antagonist blocked the effects of high dose APO (blocks increased seminal emissions) [Hull et al., 1992]. Thus, studies investigating rats suggest that D1 receptors in the mPOA promote the onset of erections and thus facilitate the initiation of male copulatory behavior while D2 receptors inhibit this aspect of male copulation.
Similar to the results in rat studies, experiments in Japanese quail using systemically administered D1 and D2 agonists and antagonists concluded that male copulatory behavior in quail is stimulated by dopamine acting on D1 receptors, but inhibited by activation of the D2 receptor subtype [Balthazart et al., 1997]. However, in contrast to rat studies, systemic injections of APO inhibits both appetitive and consummatory male sexual behaviors in a dose-dependent manner in quail [Absil et al., 1994; Castagna et al., 1997]. Furthermore, intracerebroventricular (ICV) injections of APO or the endogenous ligand, dopamine (DA), have also been shown to inhibit male sexual behavior in quail [Cornil et al., 2005b]. Thus, since APO is both a D1 and D2 receptor agonist and inhibits male sexual behavior in quail as does the administration of dopamine itself, one possible explanation of this discrepancy in the effectiveness of these ligands between the species is that APO and DA may somehow activate D2 receptors to a greater extent in quail than in rats producing an inhibition of male-typical sexual behavior.
There are some indications based on autoradiographic studies that, in the basal ganglia, D2-like receptors are present at higher densities in the avian brain than D1-like receptors in contrast to what has been reported in mammals (e.g., [Richfield et al., 1987]). Differences in the dopaminergic control of male sexual behavior in birds and mammals are most apparent when non-selective receptor agonists such as APO, are administered [Balthazart et al., 1997; Cornil et al., 2005b]. Thus, one possible explanation for differences in the action of these ligands on the regulation of male sexual behavior in birds and mammals could be due to the fact that there is a differential relative abundance of D1-like and D2-like receptors in the two taxa [Balthazart et al., 1997; Castagna et al., 1997; Balthazart et al., 2002].
The general organization of the dopamine system (e.g. anatomical localization of the cell bodies, projections, and distribution of the receptor subtypes) is similar in birds and mammals [Smeets and Reiner, 1994], but the possibility that the relative distribution and/or density of binding sites is different between birds and mammals has never been systematically studied. In vitro quantitative receptor autoradiography is an ideal method to utilize in this case because it allows the functional interaction between transmitter and receptor to be examined by localizing bound receptors. Therefore, in order to investigate the relative distribution and regional variation in the densities of D1 and D2 receptor subtypes in the Japanese quail and rats, D1-like and D2-like in vitro quantitative receptor autoradiography was performed on both species at the same time.
MATERIALS AND METHODS
2.1. Subjects and tissue preparation
Eight sexually mature male Japanese quail (Coturnix japonica) and 8 sexually mature male Long-Evans rats were obtained from CBT Farms (Chestertown, MD) and Charles River Laboratories as adults, respectively, and kept in individual cages with food and water available ad libitum. Birds and rats were housed, manipulated, and killed by using procedures approved by the IACUC at Johns Hopkins University.
Subjects were killed by decapitation and their brains quickly removed and frozen on powdered dry ice. Frozen brains were cut on a cryostat and coronal sections (16 µm) collected from the Septopalliomesencephalic tract through the Third Nerve in quail and from the Caudate Putamen to the Pineal Recess in rats. Adjacent sections were thaw-mounted on gelatin-coated slides (8–12 sections per slide), dried at room temperature, and stored at −70°C until receptor binding assays are performed. The sampling protocol for brain sections from each subject used in the assay consisted of using four adjacent slides (two for determination of total binding of D1-like and D2-like receptors, and two for determination of non-specific binding of D1-like and D2-like receptors), then skipping two sections and repeating this sampling procedure. Thus, for example, two adjacent samples on one slide refer to sections 1 and 7 for each brain. Refer to Table 1 for the detailed sampling procedure.
Table 1.
Sampling Procedure for Rat and Quail samples
| Rat | Quail | ||
|---|---|---|---|
| CPu | 10 samples over the entire rostro-caudal extent, each section 288µm apart |
MSt | 8 samples over the entire rostro-caudal extent, each section 288µm apart |
| ACb.c | 6 samples beginning at the corpus callosum, each section 96µm apart |
LSt | 5 samples over the entire rostro-caudal extent, each section 288µm apart |
| ACb.s | 6 samples beginning at the corpus callosum, each section 96µm apart |
OTu.m | 7 samples over the entire rostro-caudal extent, each section 288µm apart |
| Tu | 8 samples over the entire rostro-caudal extent, each section 288µm apart |
OTu.l | 7 samples over the entire rostro-caudal extent, each section 288µm apart |
| mPOA | 3 samples, each section 96µm apart |
mPOA | 3 samples, each section 96µm apart |
CPu, caudate putamen; ACb.c, nucleus accumbens core; ACb.s, nucleus accumbens shell; Tu, olfactory tubercle; mPOA, medial preoptic area; MSt, medial striatum; LSt, lateral striatum; OTu.m, medial olfactory tubercle; OTu.l, lateral olfactory tubercle.
2.2. Drugs
[3H]SCH 23390 (Perkin-Elmer: Specific activity = 85 Ci/mmol) and [3H]Spiperone (Amersham: Specific activity = 101 Ci/mmol) were used as labeled ligands for the D1-like and D2-like receptors, respectively. (+)butaclamol HCl (a general D1/D2 antagonist) and haloperidol (a D2-specific antagonist) were purchased from Sigma-Aldrich and were used as the non-specific cold competitors for the D1-like and D2-like receptor binding, respectively. Preliminary binding studies revealed that these two ligands provided the best signal to noise ratio.
2.3. Quantitative receptor autoradiography
The labeling of D1-like and D2-like receptors was adapted from previously described procedures [Dawson et al., 1986; Ball et al., 1995; Gallagher et al., 1999; Levens et al., 2000]. We ran preliminary autoradiographic studies to insure that we had a high specific/non-specific ratio of binding under the conditions we adopted and with the cold competitors we selected. Based on these studies, the concentrations we selected are similar to those used for these ligands in previous studies in quail and rats [Ball et al., 1995; Gallagher et al., 1999]. A 50 mM Tris-HCl (pH 7.4), 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2 buffer was used for both labelings. Slides were first fan-dried for 30 min. Next, they were preincubated in buffer at room temperature for an additional 30 min. Then, slides were incubated at room temperature for 60 min in 2.0 nM [3H]SCH 23390 in buffer for D1-like binding or 0.4 nM [3H]Spiperone for D2-like binding. This was followed by two 5 min washes in ice cold buffer and a final dip in ice cold distilled water before fan-drying. Non-specific binding for the D1-like slides was estimated by incubating adjacent sections in a medium containing 2.0 nM [3H]SCH 23390 and a cold competitor, (+)butaclamol HCl (10 µM). For D2-like slides, non-specific binding was estimated by incubating adjacent sections in a medium containing 0.4 nM [3H]Spiperone and a cold competitor, Haloperidol (10 µM). The apparent dissociation constant (Kd) for these two receptor subtypes in both species are similar (e.g., D1 quail: 1.88 nM [Ball et al., 1995]; D1 rat: 1.86 nM [Dawson et al., 1986]; D2 quail: 0.08 nM [Levens et al., 2000]; D2 rat: 0.15 nM [Levens et al., 2000]). We therefore used a similar concentration of ligand for both receptor subtypes to label the receptors. However, the maximum number of binding sites (Bmax) is higher in rats than in quail (e.g., D1 quail: 7.29 fmol/slice [Ball et al., 1995]; D1 rat: 72.91 fmol/mg tissue [Dawson et al., 1986]; D2 quail: 0.39 fmol/mg tissue [Levens et al., 2000]; D2 rat: 19.3 fmol/mg tissue [Levens et al., 2000]) so the optimal exposure time is lower in rats than in quail. Therefore the slides were exposed to Kodak® Biomax MR for 6 weeks for the quail tissue and 4 weeks for the rat tissue.
2.4 Analysis of the Autoradiograms
Digitized autoradiograms were analyzed with ImageJ (version 1.37v, Wayne Rasband, NIH, Bethesda, MD). All binding data was determined from film density in areas of interest. Tritium standards (American Radiolabeled Chemicals Inc. St. Louis, MO) were co-exposed with the slides and used to convert optical density to fmol of tritiated ligand specifically bound per mg of tissue.
Specific binding in areas of interest were defined as the density of binding in the absence of competitor (total binding) minus binding in the presence of butaclamol (for D1-like labeling; non-specific binding) or haloperidol (for D2-like labeling; non-specific binding). For each nonspecific binding slide, two sections were quantified and the average value of non-specific binding was used. To find the relative density of D2 to D1 receptors, the specific binding of D2 was divided by the specific binding of D1 for each region in each subject. The mean value of specific binding for each brain region in each subject was found and the ratio of D2 to D1 receptors was analyzed.
In preparing the manuscript, the brightness (+20% for all quail images) and contrast (+30% for all images) of Figures 1 through 3 in this report were adjusted to help the reader visualize the specific binding. No adjustments were made to the images for quantification.
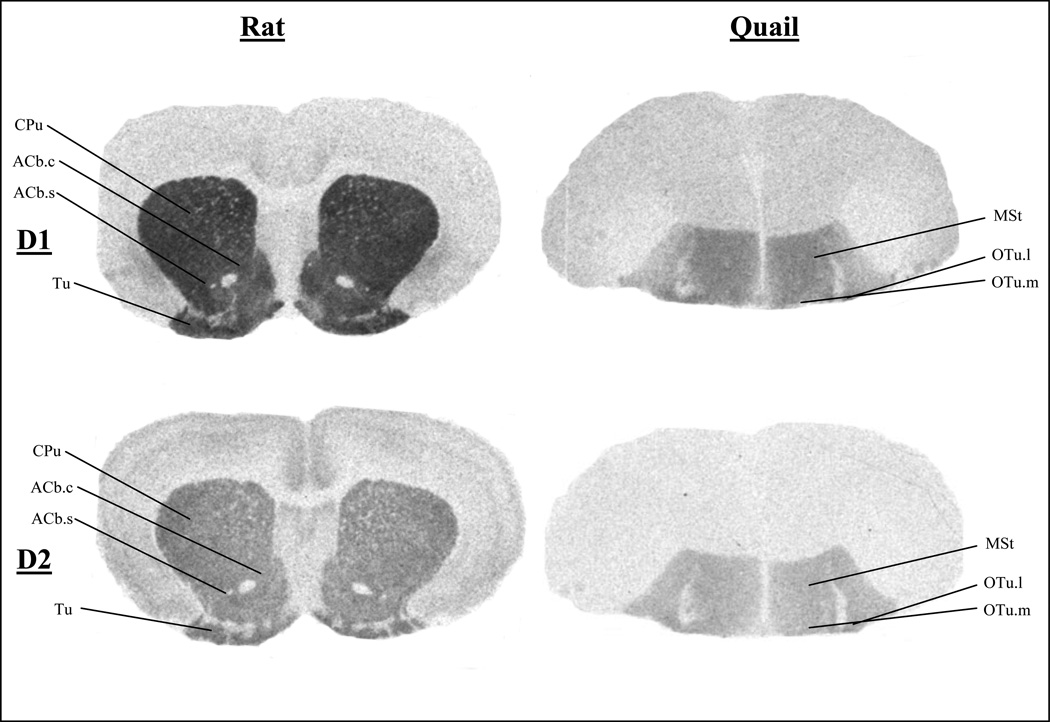
Figure 1.
Total Binding of D1-like and D2-like Receptors in Rats and Quail at the Level of the Nucleus Accumbens and Olfactory Tubercle
CPu, caudate putamen; ACb.c, nucleus accumbens core; ACb.s, nucleus accumbens shell; Tu, olfactory tubercle; MSt, medial striatum; OTu.m, medial olfactory tubercle; OTu.l, lateral olfactory tubercle.
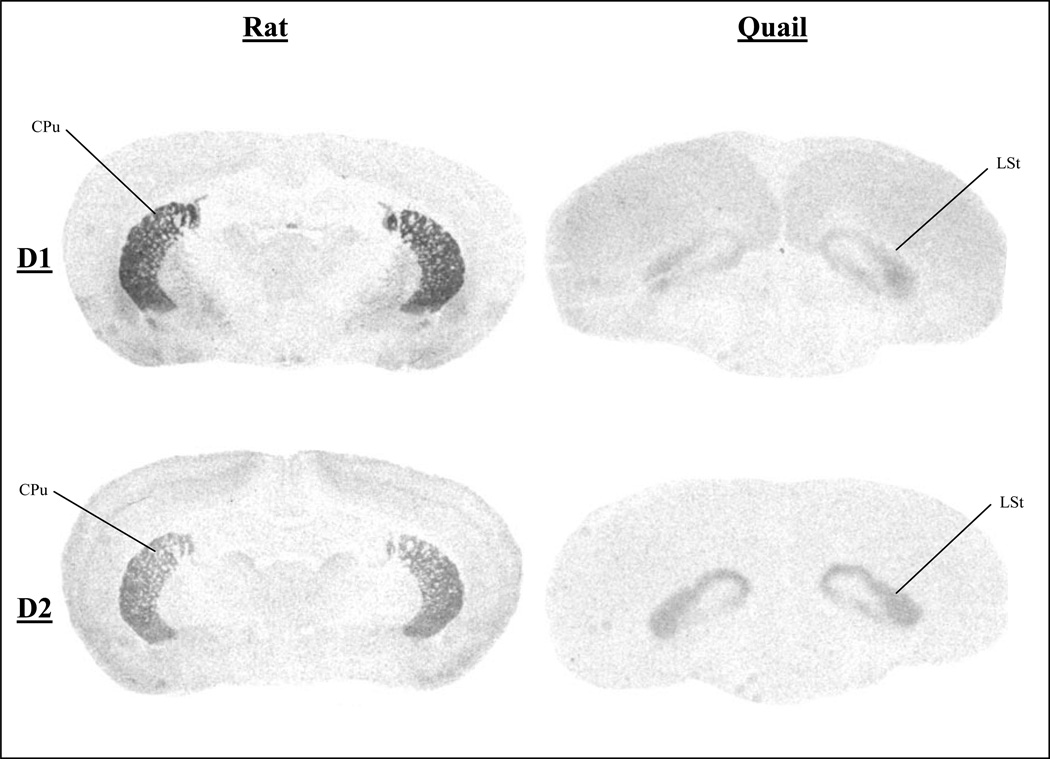
Figure 3.
Total Binding of D1-like and D2-like Receptors in Rats and Quail in Sections Caudal to the Anterior Commissure
CPu, caudate putamen; LSt, lateral striatum.
2.5. Brain Region Comparisons
The overall organization of the dopaminergic system in birds and mammals is quite similar [Smeets and Reiner, 1994]. In many cases specific homologies between areas expressing dopaminergic cells and those receiving dopaminergic input have been established [Jarvis et al., 2005; Reiner et al., 2005]. However, in some cases the exact correspondence between homologous brain structures is difficult to ascertain. For purposes of this report, the following brain regions between rats vs. quail were compared: rat caudate putamen vs. quail medial striatum (MSt) and lateral striatum; rat nucleus accumbens core and shell vs. quail MSt; rat olfactory tubercle vs. quail medial and lateral olfactory tubercle; rat mPOA vs. quail mPOA. Thus, seven independent comparisons were made. These comparisons are, in most cases, brain regions where there is strong consensus for the compared structures being homologous [Smeets and Reiner, 1994; Reiner et al., 2004; Jarvis et al., 2005]. The discussion section of this report expands on our justifications for considering these particular regions as being homologous.
2.6. Statistical Analyses
All results are expressed as means ± standard error of the mean (SEM). A T-test was used to uncover the between subject effect of species on the overall D2 to D1 ratio. The D2 to D1 ratio comparisons between similar brain regions were analyzed by independent T-tests, and because multiple comparisons result in an inflation of the Type I error rate (inflated alpha), a Bonferroni correction procedure was implemented. Differences in D2 to D1 ratio were therefore considered significant for p<0.007 (Bonferroni corrected). All analyses were carried out with Windows version of the software SPSS, version 16.0, and the neuroanatomical nomenclature for quail used in this report is based on the revised nomenclature for avian brain [Reiner et al., 2004].
RESULTS
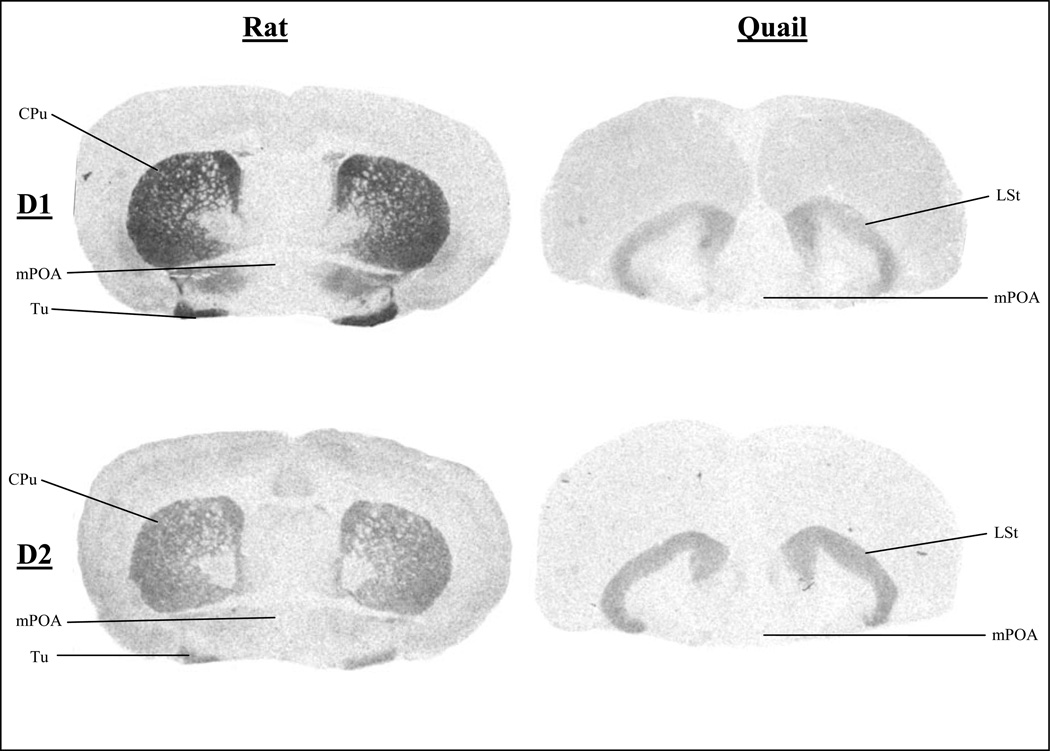
Figures 1, 2, and 3 present autoradiograms showing the total binding of D1-like and D2-like receptors in rat and quail at levels in the brain that illustrate homologous regions. These autoradiograms clearly show the different ratio of D2-like to D1-like receptors of homologous regions throughout the brain, such that quail have a higher ratio of D2-like receptors as compared to rats.
Figure 2.
Total Binding of D1-like and D2-like Receptors in Rats and Quail at the Level of the Anterior Commissure
CPu, caudate putamen; Tu, olfactory tubercle; mPOA, medial preoptic area; LSt, lateral striatum.
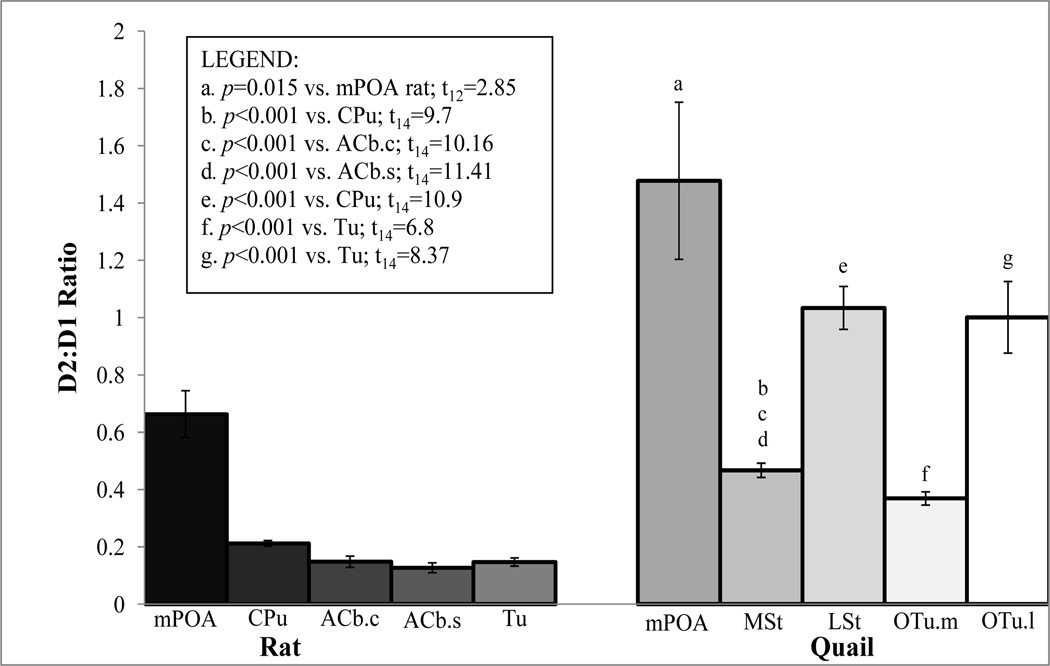
The specific binding (fmol/mg of tissue) of [3H]SCH 23390 to D1-like receptors and [3H]Spiperone to D2-like receptors in five brain regions for both rat and quail are shown in Tables 2 and 3. As expected, the results show that the absolute number of D1-like and D2-like receptors across the selected brain regions varies among species such that rats show a trend of having a greater amount of these receptors as compared to quail. When comparing the ratio of D2-like to D1-like receptors in similar brain regions across species, we found that this ratio is significantly higher in quail than in rats, t14=8.61, p<0.001 (see Figure 4 for mean ratio ± SEM rat vs. quail and the Legend for statistical comparisons among brain regions). The mPOA difference does not reach significance when corrected for multiple comparisons with Bonferroni procedures, but shows a trend that is consistent with the other regions (uncorrected p=0.015).
Table 2.
Mean Specific Binding of [3H]SCH 23390 to D1 receptors and [3H]Spiperone to D2 receptors and Ratio of D2:D1 receptors in Quail
| D1 binding | D2 binding | D2:D1 Ratio | |
|---|---|---|---|
| MSt | 196.10 ± 13.34 | 90.82 ± 6.02 | 0.467 ± 0.025 |
| LSt | 76.69 ± 4.88 | 77.66 ± 4.47 | 1.034 ± 0.075 |
| OTu.m | 153.74 ± 9.17 | 56.35 ± 4.60 | 0.369 ± 0.023 |
| OTu.l | 133.22 ± 19.21 | 119.53 ± 9.33 | 1.001 ± 0.125 |
| mPOA | 5.36 ± 1.67 | 6.23 ± 1.09 | 1.478 ± 0.274 |
All values are mean fmol/mg of tissue ± 1 SE, n=8 per group except mPOA where n=7.
MSt, medial striatum; LSt, lateral striatum; OTu.m, medial olfactory tubercle; OTu.l, lateral olfactory tubercle; mPOA, medial preoptic area.
Table 3.
Mean Specific Binding of [3H]SCH 23390 to D1 receptors and [3H]Spiperone to D2 receptors and Ratio of D2:D1 receptors in Rats
| D1 binding | D2 binding | D2:D1 Ratio | |
|---|---|---|---|
| CPu | 685.12 ± 33.88 | 146.67 ± 12.42 | 0.212 ± 0.010 |
| ACb.c | 611.21 ± 36.39 | 87.34 ± 8.93 | 0.148 ± 0.020 |
| ACb.s | 748.74 ± 43.83 | 91.51 ± 9.65 | 0.127 ± 0.017 |
| Tu | 755.74 ± 50.82 | 110.82 ± 11.58 | 0.147 ± 0.014 |
| mPOA | 11.23 ± 7.27 | 7.907 ± 5.43 | 0.663 ± 0.082 |
All values are mean fmol/mg of tissue ± 1 SE, n=8 per group except mPOA where n=7.
CPu, caudate putamen; ACb.c, nucleus accumbens core; ACb.s, nucleus accumbens shell; Tu, olfactory tubercle; mPOA, medial preoptic area.
Figure 4.
Ratio of D2 to D1 receptors of Various Brain Nuclei in Rats and Quail
Values are mean ratio of D2-like to D1-like receptors ± 1 SE, n=8 for each group except mPOA where n=7.
CPu, caudate putamen; ACb.c, nucleus accumbens core; ACb.s, nucleus accumbens shell; Tu, olfactory tubercle; mPOA, medial preoptic area; MSt, medial striatum; LSt, lateral striatum; OTu.m, medial olfactory tubercle; OTu.l, lateral olfactory tubercle.
DISCUSSION
These autoradiographic labeling studies revealed a difference in the relative density of D1-like and D2-like receptors in brains of quail and rats. Overall, there was a greater ratio of D2-like to D1-like receptors in quail as compared to rats, and this trend remained for each of the seven brain region comparisons investigated. This finding is consistent with a previous comparative study byRichfield et al. (1987) where it is suggested that there is such a difference in relative receptor densities in birds and mammals for D1-like and D2-like receptor densities in the basal ganglia of rats as compared to pigeons. In our study, we investigated such possible taxonomic differences in receptor densities in a more systematic manner.
One challenge faced in such a comparative analysis is ensuring that our comparisons are of homologous areas. We quantified structures in the telencephalon, diencephalon and mesencephalon that are well known to be dopaminoceptive and/or are of functional interest to us because of the role they play in control of sexual behavior. In most cases, the comparisons were straightforward because homologies between birds and mammals in structures that synthesize dopamine or receive dopamine inputs are well established [Smeets and Reiner, 1994; Reiner et al., 2004]. However, in some cases, the precise regions selected for quantification were also influenced by distinctions developed in previous autoradiographic studies conducted on quail and rats. For example, it has previously been reported in quail that there is a different density of D1-like receptors in the medial as compared to the lateral striatum as well as in the medial as compared to the lateral olfactory tubercle [Ball et al., 1995], therefore separate measurements were taken for these regions. Together, the caudate nucleus and the putamen (CPu) make up the dorsal striatum in rats [Butler and Hodos, 2005], so separate measurements were taken for the medial and lateral regions of the striatum in quail and compared to single values of the CPu in rats so that homologous structures in the two species could be compared. Similarly, separate measurements were taken for the medial and lateral olfactory tubercle in quail (which can be distinguished based on differences in D1 receptor density; [Ball et al., 1995; Mezey and Csillag, 2002; Bálint and Csillag, 2007]) and compared to single measurements of the olfactory tubercle in rats where such a distinction is not apparent.
The nucleus accumbens also had to be handled differently in rats versus quail. In our study, this nucleus is clearly discerned from surrounding striatal structures in rats and furthermore, the nucleus accumbens core and shell can also be discerned and were quantified separately. In quail and other birds, the striatal region that includes the nucleus accumbens exhibits a high dopamine receptor binding density but there is no clear difference in binding density between the nucleus accumbens and the surrounding MSt. To confirm this, we quantified the ratio of D2 to D1 receptors in the ventromedial MSt as that contains the avian homologue to the nucleus accumbens and compared via paired samples T-test to the entire MSt. No significant difference between these regions was found (t7=0.474, p=0.65). This observation is consistent with previous anatomical studies in birds indicating that the nucleus accumbens is adjacent to and encompassed by the MSt but in many cases based on chemical neuroanatomical studies it has no apparent distinct discernable histological boundaries with the MSt [Mezey and Csillag, 2002; Bálint and Csillag, 2007]. However, hodological studies combined with a suite of neurohistological markers have allowed investigators to identify an accumbens like structure in the MSt of birds that is thought to be homologous to the mammalian accumbens [Mezey and Csillag, 2002; Bálint and Csillag, 2007]. Thus the nucleus accumbens comparisons between quail and rat compares mean values for the MSt in quail to the nucleus accumbens core or shell in rats.
The present results suggesting variation in receptor expression could contribute to an explanation of species variation in the responsiveness to dopamine-like compounds related to the regulation of male sexual behavior. The pharmacological characterization of the D1 and D2-like dopamine receptors from the brain of the leopard frog, Rana pipiens, indicates that in the amphibian brain dopamine receptors also fall into two subtypes as is the case in mammals. However, the binding characteristics of these two subtypes are slightly different from those described in mammals [Chu et al., 2001]. Species variation in the effectiveness of dopaminergic ligands has also been reported among lizards in the genus Cnemidophorus [Woolley et al., 2001]. In this genus there are sexually reproducing species (such as C. inornatus) that are ancestral to parthenogenetic all female species (such as C. uniparens). Male-typical sexual behaviors (e.g., mounting) can be stimulated in both species by D1 agonists but the effective dose is much lower in the parthenogenetic C. uniparens than what is needed in the sexually reproducing C. inornatus [Woolley et al., 2001]. This difference has been attributed to the fact that the all female species is triploid and because of this increased ploidy the parthenogenetic species may have a higher expression of D1 receptors in areas critical for the control of male sexual behavior.
Systemic injections of selective D1-like receptor agonists and D2-like receptor antagonists stimulate copulatory behavior in quail while the D1-like receptor antagonist and D2-like receptor agonist inhibits this behavior [Balthazart et al., 1997]. Recently, our lab replicated these effects using ICV injections, showing that the effects of administration of these compounds are a result of central dopamine receptors [Kleitz et al., 2008]. Therefore, it is reasonable to conclude that central D1 receptors facilitate male sexual behavior in quail while D2 receptors inhibit the behavior. Thus, as the current experiment unveiled, the higher ratio of D2-like to D1-like receptors in quail would more likely activate inhibitory processes in quail than in rats when administration of the non-selective dopamine agonist apomorphine (APO) is given. Therefore, our results could provide a possible explanation to the inhibition of male sexual behavior in quail treated with APO, since this compound seems to bind preferentially with the D2 receptor subtype, especially at higher doses. A similar explanation can be used to explain why ICV injections of dopamine (DA) itself inhibits male sexual behavior in quail as well [Cornil et al., 2005b].
Different dopamine cell groups facilitate different aspects of male sexual behavior in rats with action in the mPOA being particularly critical. Based on these studies, Hull and colleagues (1999) hypothesized that sensory input from a receptive female and/or the act of copulation itself elicits the release of dopamine in each of the three integrative systems: the nigrostriatal system, mesolimbic system, and the medial preoptic system. Output from these systems controls the expression of sexual motivation, genital reflexes, and somatomotor patterns of copulation. The nigrostriatal system is hypothesized to enhance both the willingness to respond to stimuli as well as motor integration, while the mesolimbic system is critical for appetitive behavior and reinforcement [Hull et al., 1999]. Dopamine in the striatum (nigrostriatal system) disinhibits pathways through which the cortex elicits movements, and it has been found that dopamine is released in the striatum only after the male begins to copulate [Damsma et al., 1992]. The substantia nigra projects to the basal ganglia (striatopallidal complex); the caudate putamen then makes reciprocal connections with the substantia nigra and the motor cortex [Butler and Hodos, 2005]. Alternatively, the mesolimbic system links the ventral tegmental area in the midbrain to the nucleus accumbens in the limbic system [Butler and Hodos, 2005]. Extracellular dopamine increases in the nucleus accumbens during precopulatory interactions as well as during copulation, suggesting that mesolimbic dopamine levels contribute to motivation of sexual behavior [Damsma et al., 1992].
Furthermore, the medial preoptic system is hypothesized to focus the male’s motivation on sexually relevant stimuli, coordinate the genital reflexes necessary for erection and ejaculation, and enhances species-typical motor patterns of copulation [Hull et al., 1999]. Located just anterior to the hypothalamus, the mPOA receives indirect sensory input from almost all sensory modalities and sends connections back to those sources [Simerly and Swanson, 1986; Panzica et al., 1996], suggesting that mPOA neurons can modify the processing of sensory stimuli. The major efferent projections of the mPOA are to hypothalamic, midbrain, and brain stem nuclei that regulate autonomic or somatomotor patterns and motivational states [Simerly and Swanson, 1988; Panzica et al., 1996].
In male rats, extracellular DA release in the mPOA seems to be essential to facilitate male sexual behavior [Hull et al., 1995], but it remains unclear what the relationship is between DA release and male sexual behavior in quail. Cornil and collaborators [Cornil et al., 2005a] conducted an experiment using quail brain tissue analyzed with high performance liquid chromatography with electrochemical detection following sexual interactions. These researchers found a significant decrease in DA activity, as assessed by the ratio of the amount of dopaminergic metabolites (3,4-dihydroxyphenylacetate [DOPAC] and homovanillic acid [HVA]) over dopamine, in the POA/hypothalamus following sexual interactions. This paper also revealed negative correlations between sexual activity and DOPAC levels and DOPAC/DA ratios. However, the nature of this relationship remains to be elucidated. It remains unclear whether changes in monoamine levels are the cause or the consequence of changes in the behavior in quail. Thus, in vivo microdialysis experiments could potentially answer some of these questions, specifically investigating the extracellular release of DA in the preoptic area of Japanese quail due to sexual motivation and performance. These experiments are essential to better understand the role of DA in male sexual behavior in quail.
ACKNOWLEDGMENTS
This work is supported by grant R01 NIH/MH50388. HKK is supported by NIH T32 HD007276. CAC is a FNRS Research Fellow. In addition, we thank Joel Gabre for helping digitize autoradiograms.
REFERENCES
- Absil P, Das S, Balthazart J. Effects of apomorphine on sexual behavior in male quail. Pharmacol Biochem Behav. 1994;47:77–88. doi: 10.1016/0091-3057(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Bálint E, Csillag A. Nucleus accumbens subregions: hodological and immunohistochemical study in the domestic chick (Gallus domesticus) Cell Tissue Res. 2007;327:221–230. doi: 10.1007/s00441-006-0295-0. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine Mechanisms Regulating Reproductive Cycles and Reproductive Behavior in Birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, editors. Hormones, Brain, Behavior. vol 2. San Diego, California: Academic Press; 2002. pp. 649–798. [Google Scholar]
- Ball GF, Casto JM, Balthazart J. Autoradiographic localization of D1-like dopamine receptors in the forebrain of male and female Japanese quail and their relationship with immunoreactive tyrosine hydroxylase. J Chem Neuroanat. 1995;9:121–133. doi: 10.1016/0891-0618(95)00075-i. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Annu Rev Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. ed 2nd. Hoboken, NJ: John Wiley & Sons, Inc.; 2005. [Google Scholar]
- Castagna C, Ball GF, Balthazart J. Effects of dopamine agonists on appetitive and consummatory male sexual behavior in Japanese quail. Pharmacol Biochem Behav. 1997;58:403–414. doi: 10.1016/s0091-3057(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Chu J, Wilczynski W, Wilcox RE. Pharmacological characterization of the D1- and D2-like dopamine receptors from the brain of the leopard frog, Rana pipiens. Brain Behav Evol. 2001;57:328–342. doi: 10.1159/000047251. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005a;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dejace C, Ball GF, Balthazart J. Dopamine modulates male sexual behavior in Japanese quail in part via actions on noradrenergic receptors. Behav Brain Res. 2005b;163:42–57. doi: 10.1016/j.bbr.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Barone P, Sidhu A, Wamsley JK, Chase TN. Quantitative autoradiographic localization of D-1 dopamine receptors in the rat brain: use of the iodinated ligand [125I]SCH 23982. Neurosci Lett. 1986;68:261–266. doi: 10.1016/0304-3940(86)90499-4. [DOI] [PubMed] [Google Scholar]
- Gallagher IM, Clow A, Jenner P, Glover V. Long-term effects of pergolide and (−)-deprenyl on 3H-mazindol and 3H-spiperone binding in rat brain. Neurobiol Aging. 1999;20:709–713. doi: 10.1016/s0197-4580(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA, Loucks JA. Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: implications for copulation. Life Sci. 1992;51:1705–1713. doi: 10.1016/0024-3205(92)90299-5. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male Sexual Behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 1. San Diego, California: Academic Press; 2002. [Google Scholar]
- Hull EM, Wood RI, McKenna KE. Neurobiology of Male Sexual Behavior. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Elsevier; 2006. pp. 1729–1824. [Google Scholar]
- Jarvis ED, Gunturkun O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz HK, Cornil CA, Balthazart J, Ball GF. Effects of central injections of a D2-like receptor agonist and antagonist on male sexual behavior in Japanese quail. Abstr.-Soc. Neurosci. 2008 doi: 10.1111/j.1460-9568.2010.07257.x. (No. 594.10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens N, Green TA, Akins CK, Bardo MT. Dopamine D(2)-like receptor binding in the brain of male Japanese quail (Coturnix japonica) Neurosci Lett. 2000;296:77–80. doi: 10.1016/s0304-3940(00)01651-7. [DOI] [PubMed] [Google Scholar]
- Mezey S, Csillag A. Selective striatal connections of midbrain dopaminergic nuclei in the chick (Gallus domesticus) Cell Tissue Res. 2002;308:35–46. doi: 10.1007/s00441-002-0514-2. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Yamamoto K, Karten HJ. Organization and evolution of the avian forebrain. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1080–1102. doi: 10.1002/ar.a.20253. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Young AB, Penney JB. Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J Comp Neurol. 1987;262:446–463. doi: 10.1002/cne.902620308. [DOI] [PubMed] [Google Scholar]
- Scaletta LL, Hull EM. Systemic or intracranial apomorphine increases copulation in long-term castrated male rats. Pharmacol Biochem Behav. 1990;37:471–475. doi: 10.1016/0091-3057(90)90015-a. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Reiner AE, editors. Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Woolley SC, Sakata JT, Gupta A, Crews D. Evolutionary changes in dopaminergic modulation of courtship behavior in Cnemidophorus whiptail lizards. Horm Behav. 2001;40:483–489. doi: 10.1006/hbeh.2001.1713. [DOI] [PubMed] [Google Scholar]