Fig. 1.
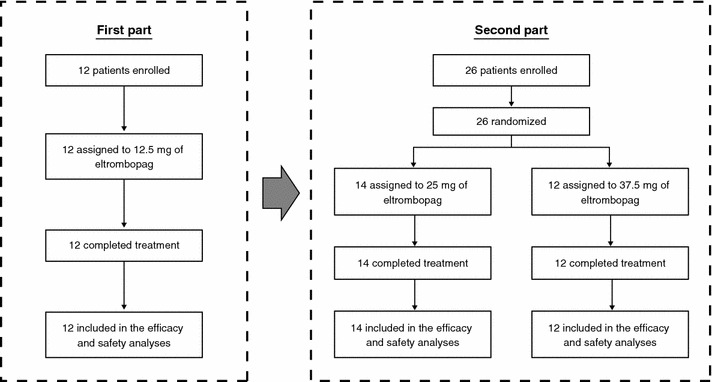
Study design. The study was a multicenter, open-label, dose-ranging phase II study that used a unique sequential design and consisted of 2 parts. After review, by a Safety Review Committee, of safety data from the 12.5 mg group (first part), new patients were randomly assigned to receive 25 or 37.5 mg of eltrombopag once daily for 2 weeks in the second part
