Figure 1.
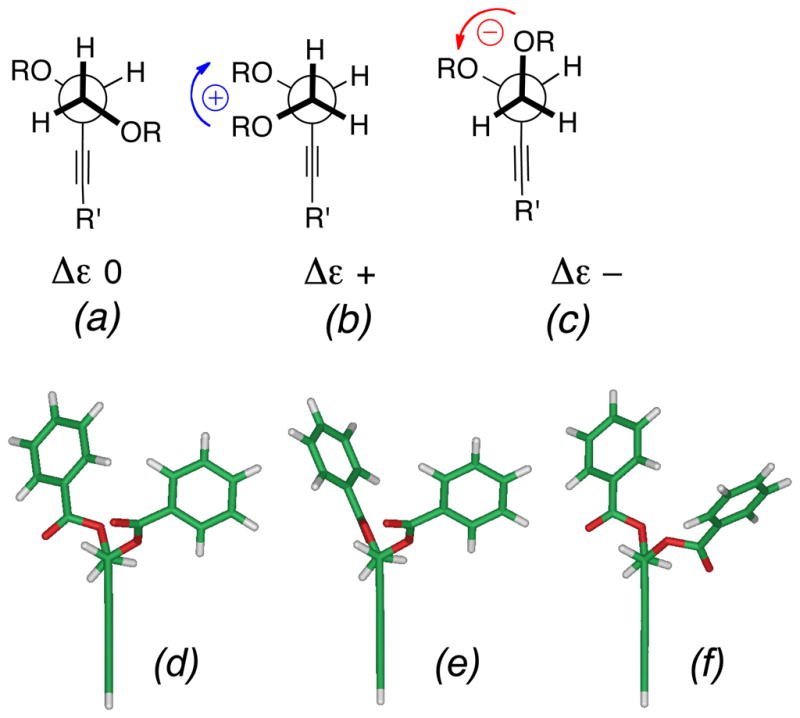
Conformers of propargylic 1,2-glycol O-dibenzoate analogs of 5. (a)–(c) idealized geometries, helicities and signs of contributions to ECCD (Δε). (d)–(f) Calculated conformers of propargylic 1,2-glycol O-dibenzoate analogs of 5 ranked in relative energy, E (kcal.mol−1), θ, the dihedral angle of the C-O bond vectors, and % Boltzmann populations (Spartan 08, semi-empirical PM3, gas phase) (d) 0, −55.6°, (22.3%) (e) 1.29, −84.1°, (13.3%) (f) 1.67, −55.0° (11.4%).. Only the three best ranked conformations are shown (see text). For ease of calculation, the chain is truncated to a 3,5-diyne.
