Abstract
The inhibition of protein tyrosine phosphatase 1B (PTP1B) is considered a valid strategy to combat insulin resistance and type II diabetes. We show here that a dichloromethane extract of Ratanhiae radix (RR_ex) dose-dependently inhibits human recombinant PTP1B in vitro and enhances insulin-stimulated glucose uptake in murine myocytes. By determination of the PTP1B inhibiting potential of 11 recently isolated lignan derivatives from RR_ex, the observed activity of the extract could be partly assigned to ratanhiaphenol III. This compound inhibited PTP1B in vitro with an IC50 of 20.2 μM and dose-dependently increased insulin receptor phosphorylation as well as insulin-stimulated glucose uptake in cultured myotubes. This is the first report to reveal an antidiabetic potential for a constituent of rhatany root, traditionally used against inflammatory disorders, by showing its capability of inhibiting PTP1B.
Keywords: PTP1B inhibition, Ratanhiae radix, Krameria lappacea, Krameriaceae, ratanhiaphenol III
Treatment and prevention of type 2 diabetes mellitus (T2DM) with its severe mostly hyperglycemia-induced micro- and macrovascular complications are major challenges of the present age [1]. Aberrantly high levels of visceral fat and subsequently acquired insulin resistance often precede the onset of T2DM. Currently used insulin sensitizers still have many side effects including weight gain or bone loss [2]. An optimal treatment for T2DM would consist of an increase in insulin sensitivity and weight loss. Protein tyrosine phosphatase 1B (PTP1B) [3] is a promising drug target since it negatively regulates insulin and leptin signalling, and its inhibition therefore leads to increased insulin sensitivity as well as higher energy expenditure, less food intake, and less weight gain [4-6].
In the course of a previous project focussing on constituents of Ratanhiae radix with antiphlogistic properties we have isolated and identified several benzofuran derivatives with marked anti-inflammatory activity in vitro and in vivo and provided a molecular explanation for the traditional use of this herbal drug against oropharyngeal inflammation [7]. Since chronic inflammation contributes to and, directly or indirectly, shares common molecular hubs with obesity and diabetes, we were prompted to investigate whether a lipophilic CH2Cl2 (DCM) extract of Ratanhiae radix (RR_ex) as well as the previously isolated lignan derivatives [7] also exert a beneficial impact on metabolism. Of note, several benzofuran derivatives have already been reported as PTP1B inhibitors (e.g., [8]). We therefore examined the PTP1B inhibiting potential of RR_ex and the lignans in vitro and whether they would enhance insulin signalling and glucose uptake in a cell-based model.
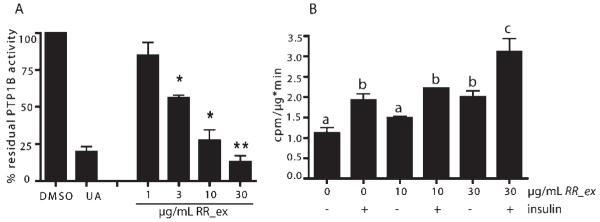
In Fig. 1A we show that RR_ex (please see Fig. 1S in Supporting Information for an HPL chromatogram of the extract) concentration-dependently (3-30 μg/mL) reduced human recombinant PTP1B activity. A known natural product-derived PTP1B inhibitor, ursolic acid (UA, 10 μM) [9], was used as a positive control in this colorimetric in vitro enzyme assay. The same extract at a concentration of 30 μg/mL was able to significantly increase glucose uptake into murine C2C12 myocytes both in the absence and presence of insulin (Fig. 1B).
Fig. 1.
A DCM extract of Ratanhiae radix inhibits PTP1B in vitro and elevates glucose uptake into C2C12 myocytes. A Different concentrations of a DCM extract of Ratanhiae radix (RR_ex) were subjected to a PTP1B in vitro enzyme assay. Ursolic acid (UA, 10 μM) was used as a positive control. Enzyme activity in the solvent (DMSO) control was set at 100%; n = 3, * p < 0.05, ** p < 0.01 (one-way AN-OVA, Dunnett’s post-test vs. DMSO control). B Basal and insulin (100 nM)-stimulated 3H-deoxyglucose uptake by C2C12 myocytes was assessed after incubation of the cells with solvent, 10, or 30 μg/mL RR_ex for 2 h [n = 3, two-way ANOVA, Bonferroni’s post-test; different superscript letters represent statistically significant differences (p < 0.05) between the respective data].
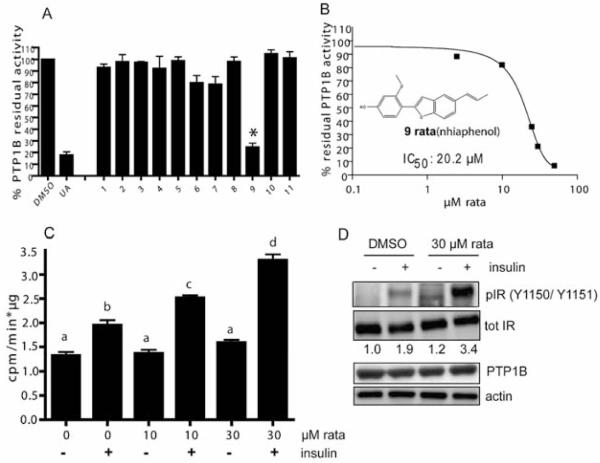
Prompted by these promising findings, we next examined eleven constituents recently isolated from RR_ex (benzofurans 1, 4-11, and 7,7′-epoxylignans 2 and 3; for structures, see Fig. 2S) [8] with regard to their capability of inhibiting PTP1B. At a concentration of 30 μM, only one compound, namely ratanhiaphenol III [compound (cpd) 9 in Fig. 2S], significantly and markedly suppressed PTP1B activity, as shown in Fig. 2A. This is surprising since several benzofuran derivatives (viz. cpds 1, 4–8, 10, 11) isolated from RR_ex just differ marginally from ratanhiaphenol III. Apparently, the isolated dihydrofurans (cpds 2 and 3) do not contribute to any PTP1B inhibiting activity. The prop-1-enyl moiety on position 5 of the 2-phenylbenzofuran skeleton seems to be one essential feature, whereas the corresponding hydroxypropyl derivatives are inactive (cpds 1 and 5). The substitution pattern of the phenyl ring obviously has a tremendous impact on the activity. A para substitution of a hydroxyl-group alone (cpd 8) does not reveal any activity; however, in combination with a methoxy-group in the ortho position (cpd 9), a distinct PTP1B inhibition could be observed. The impact of further variations on the 2-phenylbenzofuran skeleton with respect to the substitution pattern of the phenyl ring and the degree of saturation in the furan moiety remains to be clarified due to the limited number of available natural derivatives.
Fig. 2.
Ratanhiaphenol III from RR_ex inhibits PTP1B in vitro with an IC50 of 20 μM and enhances insulin-mediated glucose uptake and insulin receptor phosphorylation in C2C12 cells. A Compounds 1–11 (for structures, see Fig. 2S) from RR_ex were tested at 30 μM for their PTP1B inhibitory potential in an in vitro enzyme assay; n = 3, * p < 0.05 (one-way ANOVA, Dunnett’s post-test vs. DMSO control) as in Fig. 1. B Ratanhiaphenol III (rata) was tested in different concentrations in order to determine the IC50 value by data fitting to a sigmoidal dose response curve. C Ratanhiaphenol III (rata) was administered to serum-starved C2C12 myocytes at 10 and 30 μM for 2 h. Then basal and insulin (100 nM)-stimulated glucose (3H-DOG) uptake rates were assessed [n = 3, two-way ANOVA, Bonferroni’s post-test, different superscript letters represent statistically significant differences (p < 0.05) between the respective data]. D C2C12 myocytes were serum-starved for 4 h, treated with ratanhiaphenol III (rata; 30 μM) or DMSO for 2 h and then stimulated with 100 nM insulin for 5 min as indicated. Cell lysates were subjected to immunoblot analyses for a phosphorylated insulin receptor (pIR, Y1150/Y1151) and a total insulin receptor (IR) as well as for PTP1B, and actin as a loading control. Representative blots out of three independent experiments with consistent results are shown. The numbers below the tot IR-blot represent the mean densitometric values of the ratio pIR/tot IR (normalized to the unstimulated DMSO control) from all performed experiments.
Testing of ratanhiaphenol III in different concentrations in the in vitro PTP1B assay revealed an apparent IC50 value of 20.2 μM ( Fig. 2B). Ratanhiaphenol III was furthermore administered to C2C12 myocytes and examined concerning its influence on glucose disposal ( Fig. 2C). In concentrations of 10 and 30 μM, ratanhiaphenol III significantly boosted the insulin-stimulated glucose uptake whereas the basal glucose uptake rate was not affected. This finding is in agreement with the inhibition of PTP1B by ratanhiaphenol III and subsequent sensitization of cells for the action of insulin. The increased basal glucose uptake observed upon exposure to the extract (see Fig. 1B) apparently cannot be assigned to the action of ratanhiaphenol III and seems to be, at least partly, dependent on activation of AMP-activated kinase presumably mediated by other compounds in the extract (Fig. 3S). Inhibition of PTP1B by ratanhiaphenol III in the cell-based model is further underpinned by the increased insulin-triggered phosphorylation of the insulin receptor at tyrosines Y1150 and Y1151, sites normally dephosphorylated by PTP1B [4]. Total levels of PTP1B remain, hereby, unaffected ( Fig. 2D).
These findings demonstrate that ratanhiaphenol III is one PTP1B-inhibiting principle of RR_ex in vitro and consistently enhances the insulin response in relevant cell-based models. Our data demonstrate for the first time the beneficial potential of constituents in Ratanhiae radix, a traditionally anti-inflammatory herb, in the context of metabolic dysfunction. Its DCM extract elevates glucose disposal partly via inhibition of PTP1B by ratanhiaphenol III. Our findings encourage the examination of other herbal remedies for indications different from their traditional use in folk medicine. Such repurposing of herbal drugs is highly facilitated by the knowledge of the active compounds and/or their mode of action, as well as by the identification of common druggable molecular master switches involved in the etiology of seemingly unrelated disorders. It can be achieved by the synergy between phytochemistry and molecular pharmacology.
Materials and Methods
Ratanhiae radix, dried ground roots of Krameria lappacea (Dombey) Burdet et Simpson (syn. K. triandra Ruiz et Pavon; Krameriaceae) (500 g; KL 6269), were purchased from Mag. pharm. Kottas-Heldenberg & Sohn (Vienna, Austria) and complied with the European Pharmacopoeia. A voucher specimen (KL 6269) is deposited at the Institute of Pharmacy/Pharmacognosy, University of Innsbruck (Austria).
PTP1B enzyme was purchased from R&D Systems. Chemicals, unless stated otherwise, were obtained from Sigma-Aldrich. Mouse C212 myoblasts were from ATCC. Dulbecco’s modified essential medium (DMEM) was purchased from Lonza, and fetal calf serum, horse serum, penicillin, streptomycin, glutamine, and trypsin was from Gibco/Invitrogen. Complete™ protease inhibitor was from Roche Diagnostics, and PVDF membranes were from Bio-Rad Laboratories. The following antibodies were used: against the phosphorylated insulin receptor (at Y1150/1151), anti-total insulin receptor β, anti-PTP1B from Becton Dickinson. Secondary antibodies: goat anti-rabbit IgG was from New England Biolabs and goat anti-mouse IgG from Upstate. All solvents and additives used for analysis and isolation were of HPLC-grade and purchased from Merck. Ultrapure water was produced by a Sartorius Arium® 611 UV water purification system.
Extraction, isolation, and identification of pure compounds from Ratanhiae radix are extensively described in Baumgartner et al. [7]. Briefly, ground roots (300 g) were exhaustively extracted with DCM in a Soxhlet apparatus, yielding 16.32 g of DCM extract. Isolation of pure compounds was achieved using different chromatographic techniques, such as flash silica gel column chromatography (CC), Sephadex® LH-20 CC, high-speed counter current chromatography, as well as semipreparative HPLC. Identity of the isolated compounds was confirmed by spectroscopic and spectrometric methods (1D- and 2D-NMR, LC-MS, optical rotation). The purity of all isolated compounds was ≥ 96% as determined by HPLC. Ursolic acid was obtained from Sigma-Aldrich with a purity of ≥ 98.5% (HPLC).
PTP1B activity was determined using 2 mM para-nitrophenylphosphate (pNPP) in 50 mM MOPS, pH 6.5, as a substrate in the presence of 1 mM DTT. The known PTP inhibitor ursolic acid (UA, 10 μM) was used as a positive control. Extract and compounds were first dissolved in 100% DMSO and diluted to 1% DMSO in 100 μL overall assay volume. The inhibitory action towards PTP1B was measured in quadruplicates in a 96-well format. The reactions were subjected to kinetic absorbance readings at 405 nm for 30 min in a Tecan/SUNRISE™ photometer to assure non-saturation. Subsequently, the reaction was stopped with 10 M NaOH and the absorbance was again measured at 405 nm. C2C12 myoblasts (ATCC) were maintained under subconfluent conditions in DMEM medium supplemented with 10% FBS, glutamine, and penicillin/streptomycin under a 5% CO2 environment at 37°C. For differentiation into myotubes, cells (passage number < 15) were seeded in 24-well (assessment of glucose uptake) or 6-well (protein isolation for immunoblotting) plates and grown till confluency. Differentiation was then initiated by changing to DMEM supplemented with 2% horse serum. After 96 h, cells were considered fully differentiated and used for experiments within 2 days.
For assessment of glucose uptake, serum-starved C2C12 myotubes were treated with compounds, extracts, or solvent (DMSO, never exceeding a final concentration of 0.1%) for 2 h before they were stimulated with 100 nM insulin as indicated for 30 minutes in standard Krebs-Ringer HEPES buffer, pH 7.4. Then glucose uptake was assessed in the presence of 0.1 mM cold and 1 μCi/mL tritium-labelled 2-deoxyglucose (3H-DOG; NEN) for 10 min at room temperature. Then, cells were rinsed with ice-cold PBS and lysed with 0.05 N NaOH. Aliquots of the lysates were counted in a Perkin Elmer TriCarb 2100 liquid scintillation counter and used for determination of the protein content. The glucose uptake rate for each sample was determined as cpm/(min × μg protein).
For immunoblot analyses, cells were grown and treated as indicated in the figure legends. Protein determination, SDS-PAGE, Western blotting, and detections were performed as described previously [10]. In order to detect the phosphorylated and unphosphorylated form of a protein in one sample, usually two identical membranes were prepared in order to avoid ambiguous results due to incomplete stripping and subsequently impaired antibody binding.
At least three independent experiments (n) were performed. Data are presented as means ± standard error of the mean (SE). Statistical differences were analyzed with GraphPad Prism using one- or two-way ANOVA and Dunnett’s or Bonferroni’s multiple comparison post-test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgements
The authors thank Daniel Schachner, Hortenzia Beres, and Judith Benedics for excellent technical assistance. This work was in part supported by the Hochschuljubiläumsstiftung der Stadt Wien (project number 1755/2010 to EHH) and by the Austrian Science Fund (FWF) [NFN S10704-B03 to VMD and S10703-B03 to HS]. RJH was an Erasmus exchange student from the University of Barcelona.
Footnotes
Supporting information available online at http://www.thieme-connect.de/ejournals/toc/plantamedica
A HPL chromatogram of the DCM extract of Ratanhiae radix (RR_ex), structures of the contained isolated and tested lignans, as well as data concerning activation of AMP-activated kinase are available as Figs. 1S, 2S, and 3S, respectively.
Conflict of Interest
All authors declare no conflict of interest.
References
- 1.Unwin N, Whiting D, Gan D, Jacqmain O, Ghyoot G. IDF diabetes atlas. International Diabetes Federation; Brussels: 2009. [Google Scholar]
- 2.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZY, Lee SY. PTP1B inhibitors as potential therapeutics in the treatment of type 2 diabetes and obesity. Exp Opin Invest Drugs. 2003;12:223–233. doi: 10.1517/13543784.12.2.223. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. PTP1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem. 1997;272:1639–1645. doi: 10.1074/jbc.272.3.1639. [DOI] [PubMed] [Google Scholar]
- 5.Koren S, Fantus IG. Inhibition of the protein tyrosine phosphatase 1B: potential therapy for obesity, insulin resistance and type II diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2007;21:621–640. doi: 10.1016/j.beem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy B. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase 1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner L, Sosa S, Atanasov AG, Bodensieck A, Fakhrudin N, Bauer J, Del Favero G, Ponti C, Heiss EH, Schwaiger S, Ladurner A, Widowitz U, Della Loggia R, Rollinger JM, Werz O, Bauer R, Dirsch VM, Tubaro A, Stuppner H. Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and proinflammatory mediators in vitro. J Nat Prod. 2011;74:1779–1786. doi: 10.1021/np200343t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixit M, Saeed U, Kumar A, Siddigi M, Tamrakar AK, Srivastava AK, Goel A. Synthesis, molecular docking and PTP1B inhibitory activity of functionalized 4,5-dihydronaphtofurans and dibenzofurans. Med Chem. 2010;20:3329–3337. doi: 10.2174/157340608783331515. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Hong D, Zhou Y, Zhang Y, Shen Q, Li JY, Hu LH, Li J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and glucose uptake. Biochim Biophys Acta. 2006;1760:1505–1512. doi: 10.1016/j.bbagen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner RR, Steinmann D, Heiss EH, Atanasov AG, Ganzera M, Stuppner H, Dirsch VM. Bioactivity-guided isolation of 1,2,3,4,6-penta-O-galloyl-D-glucopyranose from Paeonia lactiflora as PTP1B inhibitor. J Nat Prod. 2010;73:1578–1581. doi: 10.1021/np100258e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.