Figure 1.
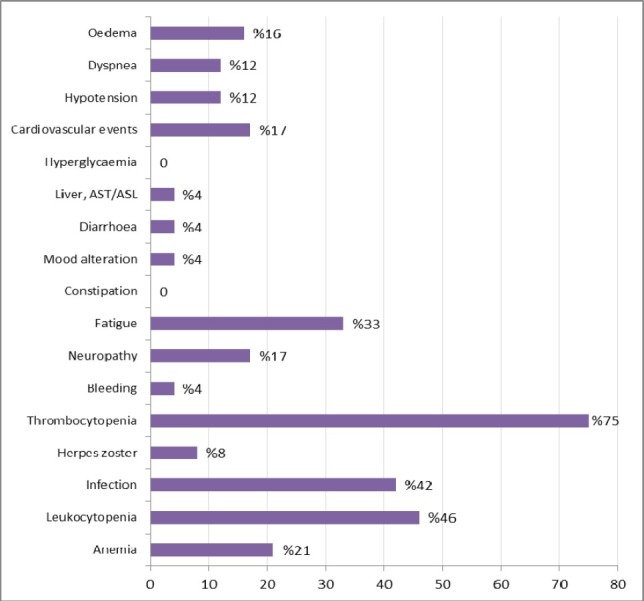
Grade 2 adverse events regardless of relation to study drug treatment (n= 24). Grade 3 adverse events were thrombocytopenia (one patient), infection (one patient), neuropathy (one patient), cardiovascular events (one patient), dyspnea (one patient) and edema (one patient).
