Abstract
Objective:
The objective of the present study was to evaluate the effect of bromocriptine on cardiovascular complications associated with type-2 diabetes mellitus (DM).
Materials and Methods:
Metabolic syndrome or type 2 DM was induced by administration of fructose (66% solution, p.o.) in rats. Bromocriptine mesylate (10 mg/kg, i.p.) was given in fructose-treated rats for a period of 6 weeks after induction of diabetes. After drug treatment, the parameters such as body weight, food and water intake, serum glucose, triglycerides, cholesterol, insulin, and blood pressure (BP) were measured weekly and at the end of study. At the end of treatment, BP was determined by invasive method and vascular reactivity was tested with adrenaline (Adr), noradrenaline (NA), and phenylephrine (PE). Acetylcholine-induced vasorelaxation was tested on isolated rat aorta and histopathology of hearts was also done.
Results:
Fructose-fed rats showed significant weight gain, hyperglycemia, hyperlipidemia, hyperinsulinemia, and rise of BP. Administration of bromocriptine at a dose 10 mg/kg, i.p. significantly decreased weight gain, serum glucose, triglyceride, cholesterol and insulin levels in rats fed on fructose. Bromocriptine also significantly reduced elevated BP in fructose-fed hypertensive rats. Chronic treatment with bromocriptine significantly improved the relaxant response to acetylcholine on fructose-fed hyperinsulinemic rat aorta and also reduced the pressor response to Adr, NA, and PE. Bromocriptine also showed a protection from hypertrophy and degenerative changes in myocardium.
Conclusion:
Bromocriptine has beneficial effect in reduction of cardiovascular complications associated with metabolic syndrome.
KEY WORDS: Bromocriptine, fructose, metabolic syndrome
Introduction
The type 2 diabetes mellitus (DM) accounts for 90 to 95% of diabetic patients.[1] It has become one of the major causes of premature illness and death, mainly due to the increased risk of cardiovascular complications which are responsible for up to 80% of deaths.[2] Unfavorable features of the type 2 DM include the triad of dyslipidemia (increased low density lipoprotein (LDL) and triglyceride (TGs) and dysregulation of glucose homeostasis damaging the vasculature through multiple mechanisms and resulting into cardiovascular complications.[3] Insulin resistance and hyperinsulinemia are the principle features of metabolic syndrome. These increased circulating levels of insulin in metabolic syndrome may lead to abnormalities in regulatory mechanisms of blood pressure (BP) and the cardiovascular complications. This can be treated better if we could avoid the “cause,” i.e., insulin resistance.[4] The anti-diabetic drugs are known to increase insulin sensitivity and can reduce hyperinsulinemia. However, a long-term treatment may cause various side effects like nephrotoxicity and hepatotoxicity.[5] At the same time, the currently available treatment for BP control may also have associated adverse effects.[6]
Bromocriptine mesylate, a D2 agonist, is approved for the treatment of type 2 DM by US-FDA in 2009.[7] However, its efficacy to reduce cardiovascular complications associated with type 2 DM are yet to be explored fully. This investigation targeted the same.
Materials and Methods
Animals
Male Wistar strain rats (150-200g) were used for the study. Animals were housed in polypropylene cages and maintained under the standard laboratory conditions (temperature 25± 2°C, 12: 12 h light-dark cycle and 50 ± 5% relative humidity with free access to food and water). Animals were acclimatized to laboratory conditions before the test. Each group consisted of five animals. All the experiments were carried out during the light period (08:00-16:00 h). The studies were carried out in accordance with the guidelines given by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India). The Institution Animal Ethical Committee of M.V.P.S College of Pharmacy, Nashik, approved the protocol (IAEC/2010/01).
Drugs and Chemicals
Metformin, pioglitazone (Glenmark Pharmaceuticals Ltd. Nasik, India) and bromocriptine mesylate (IgnataPharma Pvt. Ltd. Mumbai, India) were used. Fructose (Thermo Fisher Scientific India Pvt. Ltd, Mumbai), triglycerides, and cholesterol (Span Diagnostics Ltd, Surat) were measured using the mentioned biochemical kits. The drugs were freshly prepared in suspension of 0.5% carboxy methyl cellulose (CMC) in distilled water. All chemicals used were of analytical grade and purchased from standard manufacturers.
Fructose-induced Metabolic Syndrome in Rats
The fructose-hypertensive rat model represents an acquired form of systolic hypertension, wherein feeding a fructose-enriched diet results in hyperinsulinemia, insulin resistance, hypertriglyceridemia, and consequently hypertension.[8,9] Animals were randomly assigned to 7 groups of 5 each and were treated as follows: group I - vehicle (0.5% CMC), 0.5 ml/100 g, p.o, for 6 weeks); group II - bromocriptine (10 mg/kg i.p, for 6 weeks); group III - fructose (66% p.o, for 6 weeks); group IV - fructose + bromocriptine (fructose 66% p.o + bromocriptine 10 mg/kg, i.p for 6 weeks); group V – fructose + metformin + bromocriptine (fructose 66% p.o + metformin 350 mg/ kg, p.o + bromocriptine 10 mg/kg, i.p for 6 weeks); group VI - fructose + pioglitazone + bromocriptine (fructose 66% p.o + pioglitazone 10 mg/kg, p.o + bromocriptine 10 mg/kg, i.p for 6 weeks); group VII – fructose + insulin + bromocriptine (fructose 66% p.o + insulin 2IU, s.c + bromocriptine 10 mg/ kg, i.p for 6 weeks).
Animals were administered with fructose (66% solution, p.o, for 6 weeks) to induce metabolic syndrome or type 2 DM. Bromocriptine, metformin, pioglitazone, and insulin were administered daily for a period of 6 weeks after induction of metabolic syndrome.[8] The body weight, food and water intake in each rat were measured before start of treatment and thereafter at a weekly interval. Using tail-cuff method, systolic BP and pulse rate were recorded weekly on Power lab data acquisition system (AD Instruments). Blood samples were collected through retro-orbital plexus under ether-anesthesia weekly for determination of serum glucose and triglyceride levels. At the end of treatment, insulin levels were measured, BP was determined (by invasive method), and vascular reactivity was tested with adrenaline (Adr), noradrenaline (NA), and phenylephrine (PE). Acetylcholine-induced vasorelaxation was measured on isolated rat aorta for each group. Histopathology of hearts was also done at the end of treatment.[10,11]
The rats were anesthetized under light ether; blood was removed from the retro-orbital plexus using a capillary in micro sample tubes, serum was separated and used for following biochemical investigations: serum glucose (by standard glucose oxidase-peroxidase kit), serum triglycerides and serum insulin (by radio-immunoassay method).
Vascular Reactivity to Catecholamines
After the completion of treatment schedule, rats from each group were anesthetized with ketamine and xylazine (75 mg/kg and 15 mg/kg i.p., respectively). Right jugular vein was cannulated with fine polyethylene catheter for the administration of drugs. BP was recorded from left common carotid artery using pressure transducer by direct method on Power lab data acquisition system (AD Instruments). Heparinized saline (100 IU/ml) was filled in transducer and the fine catheter cannulated to the carotid artery to prevent clotting. After 30 minutes of stabilization, mean change in BP in response to NA (1 μg/kg), Adr (1 μg/kg), and PE (1 μg/kg) was recorded.[11]
In vitro study—Acetylcholine-induced Vasorelaxation on Isolated Rat Aorta
Immediately after completion of vascular reactivity studies, rats were sacrificed by cervical dislocation. Midline abdominal incision was made and the entire descending thoracic aorta from arch down to the diaphragm was isolated and placed in Krebs solution (composition in mM: NaCl 118.4;KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4 1.2; NaHCO3 25, glucose 11) at a temperature of 37°C and aeration with 95% O2 and 5% CO2. Connective tissue and adhering fat was removed from aorta. Rings of 3 mm length were prepared and mounted in an organ bath containing 15 ml of Krebs solution. Contractions were recorded by suspending the rings between two stainless-steel hooks, one of which was attached to the end of a bathing tube and the other to a force transducer (PowerLab, AD Instruments). Care was taken to ensure that the endothelial layer was not damaged during preparation of the aortic rings. One hook was fixed to a micrometric manipulator allowing adjustments in resting tension of the rings and the other was connected to a force displacement transducer for the measurement of isometric force. The resting tension of 1 g was applied to the preparation and equilibrated in a 15-ml bathing solution for 90 to 120 minutes before the experiment with change of solution every 15 minutes. After equilibration, the rings were exposed to 3’10-6 M PE. When the contractile response to PE was plateaued, acetylcholine was added in a cumulative fashion and vasorelaxation was recorded.[11]
Statistical Analysis
Results are expressed as mean ± SEM, and the statistical analysis of data was done using one-way analysis of variance (ANOVA) followed by Dunnett's test. Probability <0.05 was considered to be statistically significant.
Result
Body Weight, Food and Water Consumption
Fructose treatment significantly increased body weights (P<0.001) as compared to control. Administration of bromocriptine (10 mg/kg, i.p) for 6 weeks per se showed normal body weights. However, bromocriptine and its combination with metformin, pioglitazone, and insulin significantly decreased body weight as compared to fructose-treated group after 6 weeks of treatment (P<0.001) [Figure 1].
Figure 1.
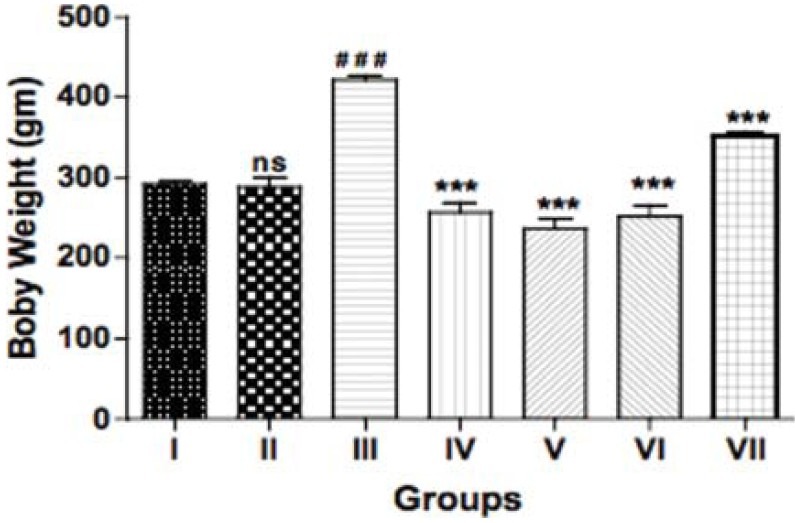
Effect of bromocriptine on body weight in rats having fructose-induced metabolic syndrome. Group I: vehicle; Group II: bromocriptine (10 mg/kg i.p); Group III: fructose (66% p.o.); Group IV: fructose + bromocriptine; Group V: fructose + bromocriptine + metformin (350 mg/ kg); Group VI: fructose + bromocriptine + pioglitazone (10 mg/ kg); Group VII: fructose + bromocriptine + insulin (2IU, s.c). Each value represents mean ± S.E.M. (n = 5). #Group II, III compared to group I. *Groups IV, V, VI, and VII compared to group III. ###,***P<0.001 (One-way ANOVA followed by Dunnett's test)
Food and water consumption was also significantly (P<0.001) increased in fructose-treated group indicating the induction of DM. Treatment with bromocriptine and its combination with other anti-diabetic drugs significantly reduced these parameters at 6 weeks (P<0.001) (Data not shown).
Serum Glucose, Triglyceride, and Cholesterol Levels
Chronic treatment with fructose resulted in a significant elevation in the serum glucose, triglyceride, and cholesterol levels (P<0.001). Administration of bromocriptine per se showed slight reduction in these levels as compared to vehicle group. Bromocriptine in fructose-fed rats resulted in a significant decrease (P<0.001). In combination with other anti-diabetic drugs also, this significant decrease in serum glucose, triglyceride, and cholesterol levels was noted (P<0.001) at 6 weeks of treatment [Table 1].
Table 1.
Effect of bromocriptine on serum glucose, triglyceride, cholesterol, and insulin levels in fructose-induced metabolic syndrome
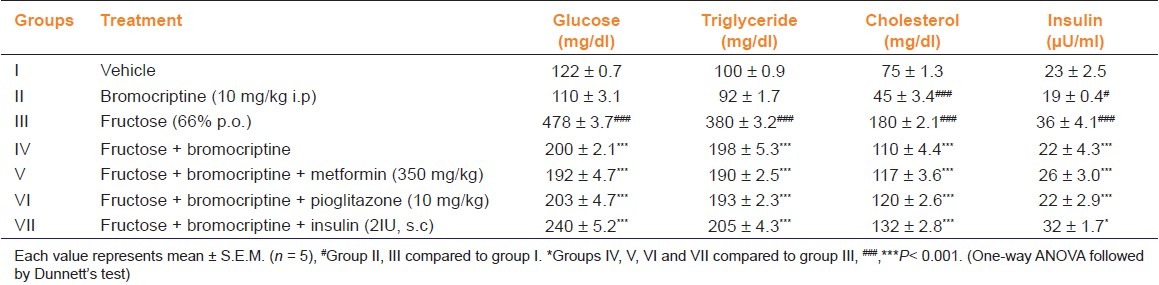
Serum Insulin Levels
Insulin levels were significantly (P<0.001) high in fructose-fed rats indicating hyperinsulinemia. The vehicle group showed normal insulin levels. Bromocriptine per se decreased the insulin level and its administration alone or in combination with anti-diabetic drugs significantly (P<0.001) reduced the insulin levels as compared with fructose-treated group [Table 1].
Heart Rate and Blood Pressure
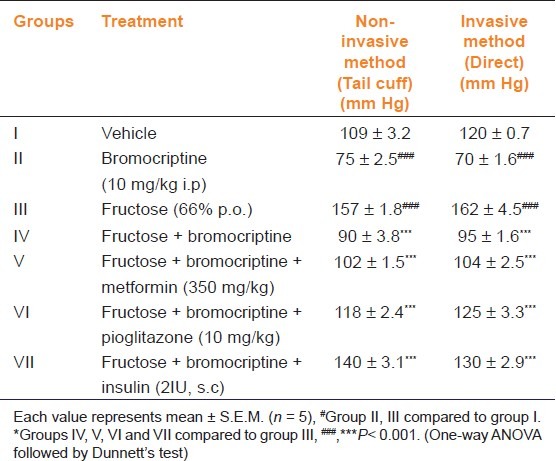
No significant changes were noticed with regard to heart rate (data not shown). When measured by the non-invasive methods, the vehicle group showed normal BP. Bromocriptine per se produced a reduction in BP as compared to vehicle group. Fructose-treated animals showed a significant (P<0.001) rise in BP. Treatment with bromocriptine or its combination with metformin, pioglitazone, and insulin significantly decreased BP as compared to fructose-treated group (P<0.001) [Table 2]. Similar results were obtained in direct measurement of BP also.
Table 2.
Effect of bromocriptine on blood pressure by non-invasive and invasive methods in fructose-induced metabolic syndrome
Vascular Reactivity
The vehicle group showed a normal vascular response to catecholamines (Adr, 1 μg/kg; NA, 1 μg/kg; and PE, 1 μg/kg), whereas fructose-treated group showed a significant (P<0.001) exaggeration in mean change in BP to these drugs as compared to the vehicle group. Chronic treatment with bromocriptine in fructose-fed group induced a significant (P<0.001) fall in mean change in BP. The bromocriptine treatment with anti-diabetic drugs also showed a significant (P<0.001) fall in mean change in BP to the catecholamines as compared to fructose-treated group [Figures 2a–c].
Figure 2.
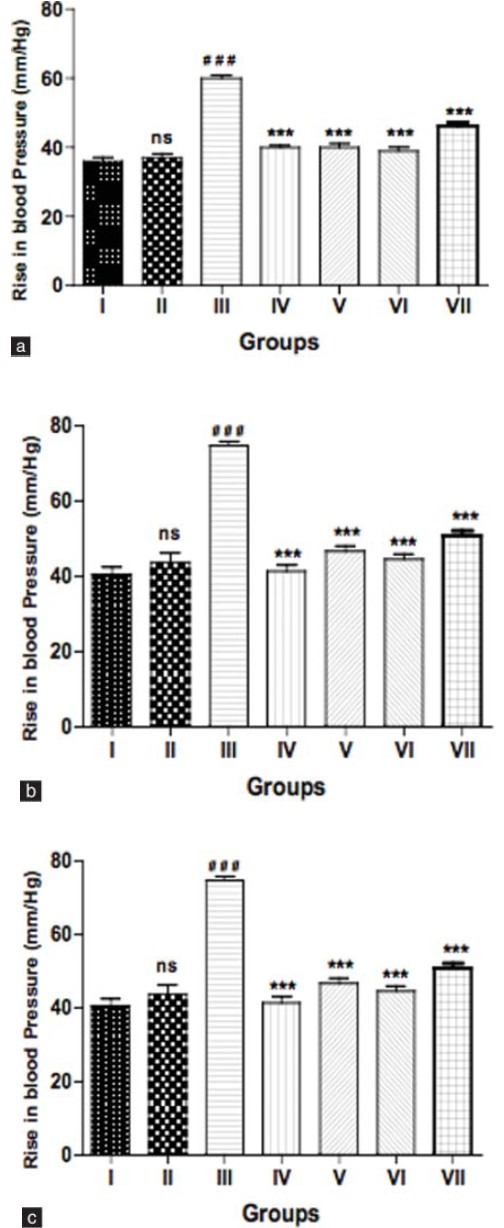
(a) Effect of bromocriptine on vascular reactivity to adrenaline (1 μg/kg), (b) noradrenaline (1 μg/kg), and (c) phenylephrine (1 μg/kg) in fructose-induced metabolic syndrome. Group I: vehicle; Group II: bromocriptine (10 mg/kg i.p); Group III: fructose (66% p.o.); Group IV: fructose + bromocriptine; Group V: fructose + bromocriptine + metformin (350 mg/kg); Group VI: fructose + bromocriptine + pioglitazone (10 mg/kg); Group VII: fructose + bromocriptine + insulin (2IU, s.c). Each value represents mean ± S.E.M. (n = 5). #Group II, III compared to Group I. *Groups IV, V, VI, and VII compared to Group III. ###,***P<0.001. (Oneway ANOVA followed by Dunnett's test)
Acetylcholine-induced Relaxation of Rat Aorta Pre-contracted with Phenylephrine (1 × 10-6M)
The aorta from vehicle group showed normal relaxant response to cumulative doses of acetylcholine (10-7M to 10-3M). This signifies normal endothelial function. Aortas from bromocriptine group also showed a normal relaxant response. However, the fructose-treated group showed a significant (P<0.001) impairment of relaxation (impaired endothelial function). Aortas from insulin-treated group showed slight improvement in relaxation after 6 weeks of treatment, while those from bromocriptine and its combination groups (metformin, pioglitazone) showed significant (P<0.01) improvement in relaxation after 6 weeks of treatment. This signifies improvement in endothelial function in rats with fructose-induced metabolic syndrome [Figure 3].
Figure 3.
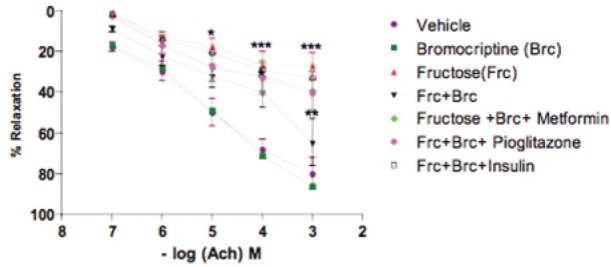
Effect of bromocriptine on acetylcholine-induced relaxation of aorta pre-contracted with phenylephrine (1 × 10-6M) in rats having fructose-induced metabolic syndrome. Group I: vehicle; Group II: bromocriptine (10 mg/kg i.p); Group III: fructose (66% p.o.); Group IV: fructose + bromocriptine; Group V: fructose + bromocriptine + metformin (350 mg/kg); Group VI: fructose + bromocriptine + pioglitazone (10 mg/kg); Group VI I: fructose + bromocriptine + insulin (2IU, s.c). Each point represents mean ± S.E.M. (n = 5). #Group II, III compared to Group I. *Groups IV, V, VI, and VII compared to Group III.#,*P<0.05, ##,**P<0.01, ###,***P<0.001. (One-way ANOVA followed by Dunnett's test)
Histopathological Examination
Heart sections (hematoxylin and eosin stained, under 10X) from vehicle and bromocriptine groups showed normal architecture of myocardial fibers. Sections from fructose-treated group revealed focal myonecrosis and lymphocytic infiltration (myocarditis). Bromocriptine and its combination groups (metformin, pioglitazone) showed better architecture as compared to fructose-treated group [Figure 4].
Figure 4.
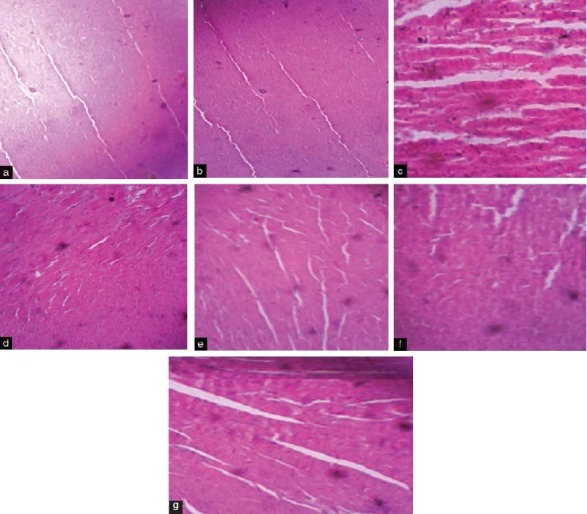
Histopathological changes in rat heart. (a) Vehicle-treated group showing normal myocardial fibers; (b) Bromocriptine group showed normal histological structure of rat heart; (c) Fructose-treated group revealed focal myonecrosis and lymphocytic infiltration (myocarditis); (d) Fructose + bromocriptine-treated rat heart showing better architecture indicating protection from fructose-induced changes; (e) Fructose + bromocriptine + metformin indicated good architecture of myocardial fibers; (f) Fructose + bromocriptine + pioglitazone showed improvement in cardiac architecture; (g) Fructose + bromocriptine + insulin showed cardiac protection
Discussion
Fructose is known to induce insulin resistance mainly in the liver.[12] In this study, rats were fed with fructose to produce several characteristic features of insulin resistance such as hyperglycemia, hyperlipidemia, and hyperinsulinemia. Oral administration of 66% fructose is reported to produce cardinal signs and characteristics of metabolic syndrome, i.e., hyperglycemia, dyslipidemia, hyperinsulinemia, and cardiovascular alterations like hypertension, endothelial dysfunction, and generalized atherosclerosis.[9] In this model, the ability of the fructose to increase BP could also be demonstrated in rats that were not genetically designed to become hypertensive. In the present investigation, it has been found that the characteristics and cardinal signs produced by oral administration of 66% fructose are similar and consistent with those reported earlier.
The results of the present study indicate that fructose-fed rats showed increased weight that may be due to an increase in the adiposity.[13] Bromocriptine showed significant weight reduction in fructose-fed rats. This was also seen in its combined use with metformin, pioglitazone, and insulin. There was significant change in food and water consumption in fructose-fed rats. This may be due to the effect of body fat on leptin receptors and in diabetic condition one of the superficial markers is excessive water consumption. Bromocriptine showed significant reduction in food and water consumption in fructose-fed rats. Decrease in food consumption may be due to its anti-appetite action.[14]
We found that in fructose-fed group, blood glucose levels were significantly (P<0.001) increased along with excessive glycosuria. Bromocriptine treatment or its combination showed a significant (P<0.001) decrease in blood glucose levels as compared to fructose-treated group after six weeks of treatment. This supports the anti-diabetic action of bromocriptine.
In type 2 diabetes, adipocytes are resistant to the action of insulin, and lipolysis continues unchecked. Increased release of free (i.e., non-esterified) fatty acids from adipocytes and their delivery to the liver provides an additional substrate for hepatic production of cholesterol and very low density lipoprotein (VLDL), which contributes to the atherosclerotic disease.[15] In this study, serum triglyceride and cholesterol levels in fructose-treated group were increased significantly (P<0.001). Bromocriptine (and its combination with other antidiabetic drugs) significantly (P<0.001) reduced the serum triglyceride and cholesterol levels in fructose-treated group. This effect may be because of the anti-steatosis action of bromocriptine.[16] At the same time, we also observed the insulin levels in fructose-treated group to be significantly higher (hyperinsulinemia). As reported earlier, in type 2 diabetes, insulin resistance develops because of a defect in insulin signal transduction mechanism.[16] So, circulating insulin concentration fails to induce glucose uptake into the cells and the hyperglycemia develops. To compensate for the hyperglycemia, pancreas secretes more insulin leading to hyperinsulinemia. Bromocriptine significantly (P<0.001) reduced the hyperinsulinemia in fructose-treated group. We observed a significant (P<0.001) increase in BP following chronic administration of fructose. An acute increase in insulin level has been shown to stimulate catecholamine secretion during euglycemic clamp studies.[17] An enhanced sympathetic activity may be involved in the development of fructose-induced hypertension. Acute increase in plasma insulin concentration has also been shown to reduce sodium excretion in dogs and human beings.[18] According to these observations, it is possible that the insulin resistance produced by high carbohydrate diet may lead to hypertension by modifying sodium balance and by enhanced sympathetic nervous system activity. Thus, hyperinsulinemia appears to play a central role in the development of carbohydrate-induced hypertension in rats and an analogous situation may also exist in human beings.
In the present study, the effect of bromocriptine was studied on various cardiovascular parameters. Heart rate was found to be lower in fructose-treated diabetic group. Chronic treatment with bromocriptine and its combination with metformin and pioglitazone restored the heart rate to near normal values. The BP was measured by indirect (tail-cuff) method and direct (carotid catheterization) methods. The results of the present study indicate that fructose-treated group had a significantly (P<0.001) higher BP after 6 weeks. Administration of bromocriptine significantly (P<0.001) reduced the elevated BP in these rats when measured by both indirect and direct methods, indicating anti-hypertensive effect. This effect may be attributed to a suppressed hyperinsulinemia.
The effect of bromocriptine was studied on the vascular reactivity to catecholamines such as Adr, NA, and PE. A significant (P<0.001) increase in pressor response to these drugs was noted in the fructose-treated group. This may be because of hypertension in fructose-treated group. Bromocriptine (its combination with metformin and pioglitazone) significantly (P<0.001) reduced this increase.
Acetylcholine induces endothelium-derived NO release in blood vessels causing them to dilate. Endothelial dysfunction is a clinical feature of metabolic syndrome as there is decreased availability of endothelium-derived NO for proper vasodilatation to take place. An impaired endothelium-mediated relaxation occurs concurrently in both human and animal models following diet-induced hyperinsulinemia.[19] We found that the relaxant response to cumulative doses of acetylcholine was significantly (P<0.001) reduced in aortas isolated from fructose-treated group. This is consistent with earlier reports indicating the presence of endothelial dysfunction. Chronic treatment with bromocriptine significantly (P<0.01) improved the relaxant response to acetylcholine in aortas isolated from fructose-treated hyperinsulinemic group.
In histopathological study, fructose-treated rat heart showed cardiac hypertrophy. Treatment with bromocriptine in fructose-fed rats showed protection from hypertrophy with much lesser degenerative changes in myocardial muscle fibers. This finding suggests an antioxidant property for bromocriptine.
We conclude that bromocriptine has beneficial effects in reduction of cardiovascular complications associated with type 2 DM. It also possesses good efficacy in combination with metformin and pioglitazone. Bromocriptine can be used for the long-term treatment of cardiovascular complications associated with type 2 diabetes mellitus.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.D’Souza A, Hussain M, Howarth FC, Woods NM, Bidasee K, Sing J. Pathogenesis and pathophysiology of accelerated atherosclerosis in diabetic heart. Mol Cell Biochem. 2009;9:148–58. doi: 10.1007/s11010-009-0148-8. [DOI] [PubMed] [Google Scholar]
- 2.Wild SH, Byrne CD. The Global burden of the metabolic syndrome and its consequences for diabetes and cardiovascular disease. In: Wild SH, Byrne CD, editors. The metabolic syndrome. 2nd ed. Chichester, UK: John Wiley and Sons Ltd; 2005. pp. 2–5. [Google Scholar]
- 3.Konrad D, Rudich A, Klip A. Insulin mediated regulation of glucose metabolism. In: Kumar S, Rahilly SO, editors. Insulin resistance: Insulin action and its disturbances in disease. 2nd ed. Chichester, UK: John Wiley and Sons Ltd; 2005. pp. 64–6. [Google Scholar]
- 4.MacLean PS, Zheng D, Jones JP, Olson AL, Dohm GL. Exercise-induced transcription of the muscle glucose transporter (GLUT 4) gene. Biochem Biophys Res Commun. 2002;292:409–14. doi: 10.1006/bbrc.2002.6654. [DOI] [PubMed] [Google Scholar]
- 5.John BB. Progressive use of medical therapies in type 2 diabetes. Diabetes Spectr. 2000;13:211–16. [Google Scholar]
- 6.Chan M. Reducing cost-related medication no adherence in patients with diabetes. Drug Benefit Trends. 2010;22:67–71. [Google Scholar]
- 7.Mahajan R. Bromocriptinemesylate: FDA-approved novel treatment for type-2 diabetes. Indian J Pharmacol. 2009;41:197–8. doi: 10.4103/0253-7613.56070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel HG. Drug discovery and evaluation: Pharmacological Assay. 2nd ed. New York, Berlin Heidelberg: Springer-Verlag; 2002. p. 176. [Google Scholar]
- 9.Hwang IS, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–6. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 10.Balaraman R, Hingorani H, Rathod SP. Studies on the antihypertensive effect of abana in rats. Indian J Pharmacol. 1993;25:209–14. [Google Scholar]
- 11.Honda H, Ushijima D. A regional variation of acetylcholine-induced relaxation in different segments of rat aorta. Physiol Behav. 1998;63:55–8. doi: 10.1016/s0031-9384(97)00388-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Choudhury RP. Vascular Biology of the metabolic syndrome. In: Krentz AJ, Wong ND, editors. Metabolic syndrome and cardiovascular disease, epidemology, assessment and management. New York: Informa Healthcare Inc; 2007. pp. 79–81. [Google Scholar]
- 13.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–22. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 14.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:1–14. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkova NB, Deedwania PC. Dyslipidemia in the metabolic syndrome. In: Krentz AJ, Wong ND, editors. Metabolic syndrome and cardiovascular disease, Epidemology, assessment, and management. New York: Informa Healthcare USA, Inc; 2007. pp. 191–6. [Google Scholar]
- 16.Davis LM, Pei Z, Trush MA, Cheskin LJ, Contoreggi C, McCullougu K, et al. Bromocriptine reduces steatosis in obese rodent models. J Hepatol. 2006;45:439–44. doi: 10.1016/j.jhep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Reaven GM, Hoffman BB. Attenuation of fructose-induced hypertension in rats by exercise training. Hypertension. 1988;12:129–32. doi: 10.1161/01.hyp.12.2.129. [DOI] [PubMed] [Google Scholar]
- 18.Defronzo RA, Goldberg M, Agus Z. The effect of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976;58:83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katakam PV, Ujhelyi MR, Hoenig ME, Miller AW. Endothelial dysfunction precedes hypertension in diet-induced insulin resistance. Am J Physiol. 1998;275:R788–92. doi: 10.1152/ajpregu.1998.275.3.R788. [DOI] [PubMed] [Google Scholar]


