Abstract
Papillary fibroelastomas are rare benign tumours of the endocardium, accounting for the most common primary valvular tumours of the heart. They typically originate from left-sided heart valves, whereas pulmonary valve involvement is anecdotal. They rarely cause valvular dysfunction, but they can cause turbulent flow and thrombus formation with consequent cerebral, retinal, coronary and pulmonary embolic disease and obstruction. We present here the case of a 56-year old man who was referred to our institution with an accidental finding, at transthoracic echocardiogram, of a mobile, pedunculated mass on the pulmonary valve, confirmed at cardiac magnetic resonance. He underwent surgical removal of the mass through median sternotomy with complete sparing of the valve. The postoperative course was unremarkable. Histopathological examination confirmed that the mass was a papillary fibroelastoma.
Keywords: Pulmonary valve, Papillary fibroelastoma, Primary cardiac tumour
INTRODUCTION
Primary cardiac tumours are a very rare pathology, with an incidence of 0.0017–0.33% at autopsy studies. The most common are myxomas (30%), followed by lipomas (10%) and papillary fibroelastomas (PFEs) (8%) [1]. These latter are rare benign tumours of the endocardium and represent the most common primary valvular tumours of the heart. Their incidence has been estimated to be between 0.00017 and 0.033% at autopsy and 0.019% in clinical series [2].
PFEs consist of small, papillary, pedunculated and avascular tumours, covered by a single layer of endothelium, containing variable amounts of elastic fibrils arranged in whorls in a hyaline stroma [3]. They typically originate from the endocardium of left-sided heart valves (95%), more frequently on the aortic (44.5%) than on the mitral valve (36.4%), whereas pulmonary valve (PV) involvement is rare, comprising 8% of the cases. Involvement of the tricuspid valve is less commonly reported. PFEs usually occur as a single tumour; however, rare cases of multiple fibroelastomas have been reported [4]. The mean age of diagnosis is 60 years [3].
It has been hypothesized that PFEs arise from the haemodynamic stress of blood flow across the endocardium, with endothelial damage followed by the deposit of fibrin fibres with thrombosis and organization [3]. They have also possibly been listed as true neoplasm, hamartomas and extensions of Lambl's excrescences. Infectious processes have been related to PFEs as a cytomegalovirus has been recovered from the tumour, suggesting a possible viral induction [5]. Histologically, PFEs consist of a dense fibroelastin core with surrounding loose connective tissue covered by a superficial endothelial layer. This connective tissue contains a mucopolysaccharide acid matrix, smooth muscle cells, collagen, elastin fibres, proteoglycans, fibroblasts, macrophages and dendritic cells. Occasionally, cysts and areas of a haemorrhage are found. Differential diagnoses include other tumours (myxomas and lipomas), thrombi and bacterial vegetations [5].
CASE REPORT
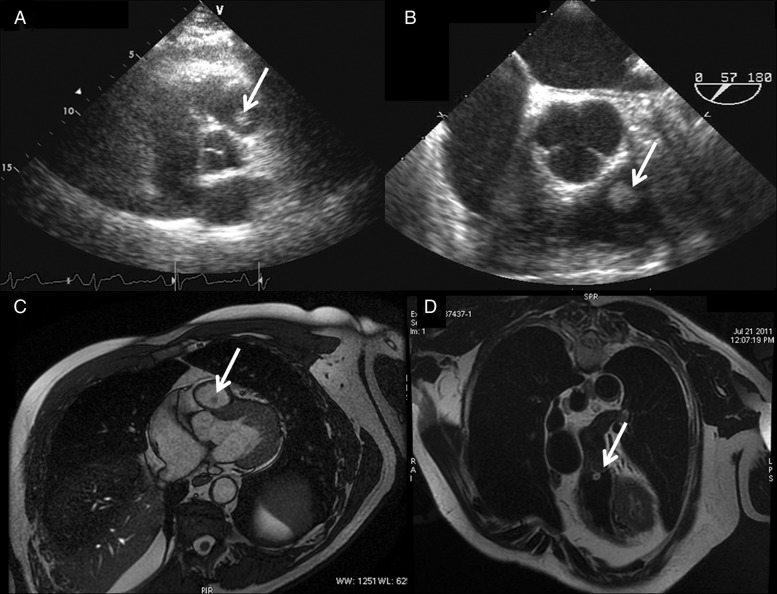
We present the case of a 56-year old, asymptomatic man who was referred to our institution with an accidental finding, at a routine transthoracic echocardiogram (Fig. 1A), of a pedunculated mass on the PV ∼13 × 9 mm in dimension. He was examined with a transoesophageal echocardiogram (Fig. 1B) that confirmed the presence of a mobile echogenic mass attached to the ventricular side of the PV anterior leaflet, likely to be a fibroelastoma. The PV itself was functionally normal. No masses were visualized on the other valves. In addition, no masses were seen in the right atrium, inferior and superior vena cavae. A cardiac magnetic resonance (CMR) was also performed with the report of a mobile, non-calcified spherical structure attached to the ventricular surface of the anterior leaflet of the PV, with intermediate signal intensity on a steady-state free precession sequence and high signal on T2-weighted sequences (Fig. 1C and D).
Figure 1:
(A and B) Transthoracic echocardiography and transoesophageal echocardiography showing the mass over the pulmonary valve (PV) (arrow). (C) Cardiac magnetic resonance (CMR) (Discovery 450, General Electric Healthcare, 1.5 T Unit, Milwaukee, WI, USA) scan images showing a spherical mass (arrow), 8×7.5 mm in dimension, attached to the anterior leaflet of the PV. The papillary fibroelastoma (PFE) appears hypointense compared with heart chambers in this steady-state free precession sequence. (D) CMR highlighting the PFE (arrow) which is hyperintense in black-blood T2-weighted coronal section.
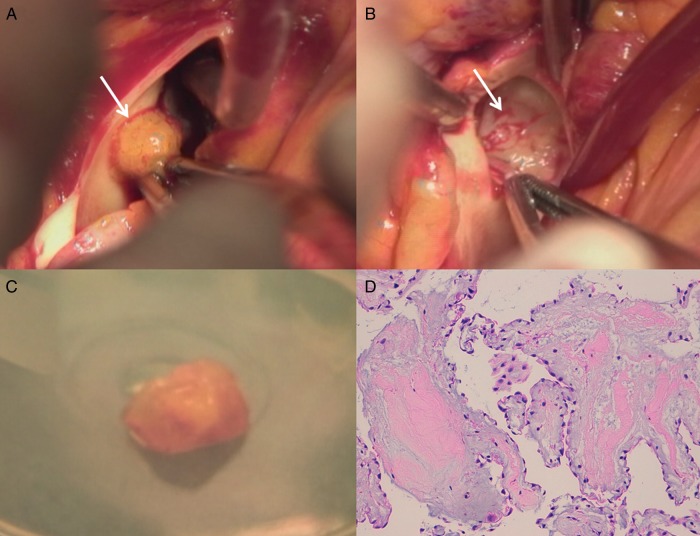
Considering the clinical condition, the age of the patient and the characteristics of the mass and its extreme mobility, we decided to remove it. Through median sternotomy, on cardiopulmonary bypass, on a beating heart with no aortic cross-clamping, we performed the excision of a gelatinous, pedunculated mass (Fig. 2A-B-C), opening the pulmonary trunk longitudinally, with complete sparing and shaving of the PV. The postoperative course was unremarkable. The patient was discharged on the 6th day in good general condition. Histopathological examination confirmed that the mass was a PFE (Fig. 2D).
Figure 2:
(A) Surgical vision of the papillary fibroelastoma (PFE) adhered to the ventricular side of the anterior leaflet of the pulmonary valve (PV). (B) Surgical view of the ventricular side of the anterior PV leaflet after the removal of the mass. (C) Vision of the mass after surgical removal: it is rounded in shape, pedunculated, semi-elastic in consistency, 1.3 cm in length. (D) Histological sample with a dense fibroelastin core surrounded by loose connective tissue covered by a superficial endothelial layer comprehensive of mucopolysaccharide acid matrix, smooth muscle cells, collagen, elastin fibres, proteoglycans and fibroblasts.
DISCUSSION
The PFE is a benign tumour with a low tendency to grow. It rarely causes valvular dysfunction [5]. However, many authors agree that PFEs have the potential to cause turbulent flow and thrombus formation, as the tumour surface comprises multiple projections and makes it highly friable as well as susceptible to clot formation [4]; consequently cerebral, retinal, coronary and pulmonary embolic disease and obstruction have been reported [3].
Because of this potential for embolic complications, the most appropriate therapy for PFEs is surgical resection. Recurrence after surgical excision has yet to be reported [3–4]. Surgery is advocated for left-sided tumours, but much less is known on the management of right-sided ones. Concerning PV PFEs, only 43 cases have been listed by Godwa et al. since 1856, autopsy series included. In 1991, Edwards et al. listed out, of 56 primary cardiac tumours, only 13 lesions affecting PV since 1931.
We know that PV PFEs are mostly asymptomatic, but they can be related to complications such as pulmonary embolism. Embolization of thrombi or tumour fragments into the pulmonary vessels has been reported [3]. In general, there is insufficient clinical experience to produce written protocols. However, we know that surgical treatment involves a relatively simple excision of the mass with excellent prognosis. Gowda et al. [3] documented a 50% PFE-related mortality rate, from either systemic embolization or outflow obstruction, in patients who did not undergo curative resection. Again, most of these patients had left-sided lesions, so it is impossible to extrapolate data solely concerning right-sided ones.
A typical diagnosis is incidental by trans-thoracic echocardiography. A subsequent transoesophageal scanning can be useful to determine the relation between the mass and the adjacent structures. Moreover, a CMR can have a leading role regarding the anatomic and tissue characterization and the functional aspect of the mass.
In conclusion, we believe that the best treatment for a PV PFE is surgical resection, which can efficiently be performed with respect to the integrity of the valve's leaflet, on-pump, with no need for aortic cross-clamp, with a cardiopulmonary bypass to drain the heart chambers. The use of long-term anticoagulation can be advocated for elderly patients with contraindications for surgery [3].
Conflict of interest: none declared.
References
- 1.Howard RA, Aldea GS, Shapira OM, Kasznica JM, Davidoff R. Papillary fibroelastoma: increasing recognition of a surgical disease. Ann Thorac Surg. 1999;68:1881–5. doi: 10.1016/s0003-4975(99)00860-7. doi:10.1016/S0003-4975(99)00860-7. [DOI] [PubMed] [Google Scholar]
- 2.Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997;30:784–90. doi: 10.1016/s0735-1097(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 3.Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404–10. doi: 10.1016/S0002-8703(03)00249-7. doi:10.1016/S0002-8703(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 4.Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001;103:2687e93. doi: 10.1161/01.cir.103.22.2687. [DOI] [PubMed] [Google Scholar]
- 5.Grandmougin D, Fayad G, Moukassa D, Decoene C, Abolmaali K, Bodart JC, et al. Cardiac valve papillary fibroelastomas: clinical, histological and immunohistochemical studies and a physiopathogenic hypothesis. J Heart Valve Dis. 2000;9:832e41. [PubMed] [Google Scholar]