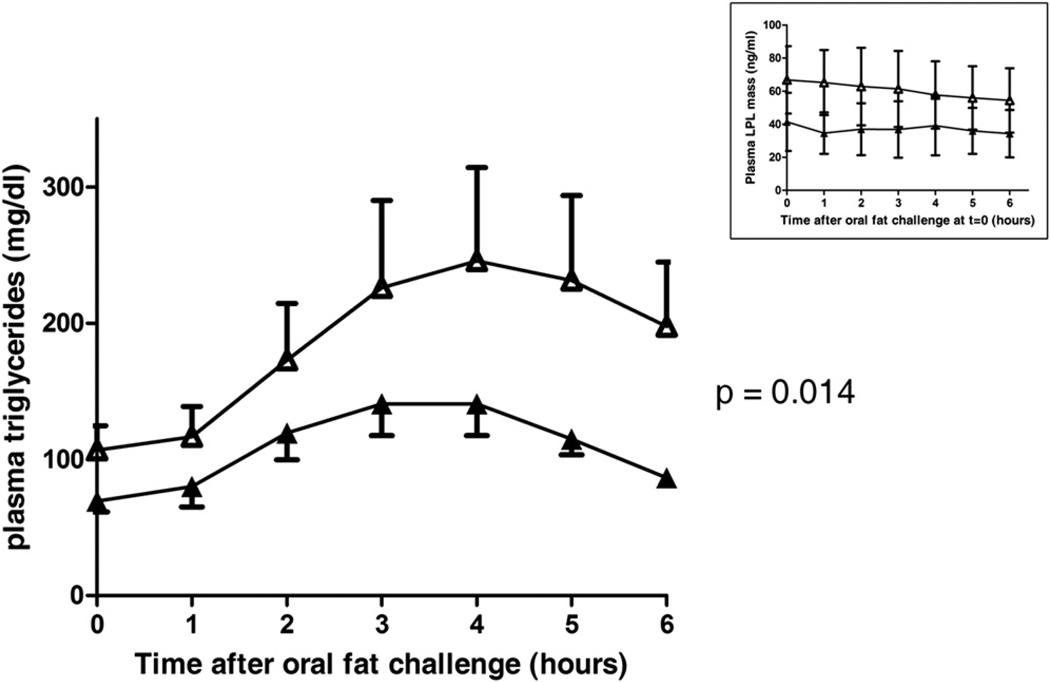
Figure 2. Carriers of the GALNT2D314A Mutation Have Improved Postprandial Plasma Triglyceride Clearance.
Depicted are plasma triglyceride levels before (t = 0) and after an oral fat challenge (t = 1–6 hr) in four carriers and four noncarriers. Compared to noncarriers (open symbols), carriers (closed symbols) have lower baseline triglyceride levels, earlier maximum triglyceride levels (t = 3 versus t = 4 in controls), and a reduced overall triglyceride increase. The estimated difference in plasma triglycerides (averaged over time) between carriers and noncarriers using a mixed linear model was 15.4 mg/dl (95% confidence interval 3.3–27.5; p = 0.014). Data are expressed as means. Error bars are SEM. The inset shows that the oral fat challenge did not change plasma LPL levels in carriers and controls when using a commercially available ELISA. The LPL levels appear lower in carriers compared to controls, but these were not significantly different from controls at any time point as assessed by unpaired Student’s t tests. Data are given as means. Error bars are SD.