Abstract
Background
Endothelial nitric oxide synthase (eNOS) has diverse roles in the female reproductive system including a role in blastocyst implantation. Aberrant expression of eNOS could therefore be significant in the pathogenesis of disorders of implantation
Materials and Methods
eNOS protein and mRNA levels in the endometrium of women with recurrent miscarriages, unexplained infertility, and a control group was determined by compartmental quantitative immunohistochemistry and real time RT-PCR
Results
eNOS was immunolocalized to all layers of the endometrium and the vascular endothelium. eNOS protein expression was higher in glandular epithelium (P=0.004) and luminal epithelium (P=0.002) but not vascular endothelium and stroma (P=0.14) in women with recurrent miscarriage. Similarly, in women with unexplained infertility eNOS expression was significantly higher (P<0.03) in luminal epithelium but not in any other compartments compared with the control group. The levels of mRNA expression as determined by real time RT-PCR confirmed the protein data demonstrating higher eNOS mRNA expression In the endometrium of women with recurrent miscarriage and unexplained infertility compared with controls
Conclusion
Increased expression of eNOS in glandular and luminal epithelium of the endometrium in women with recurrent miscarriages and unexplained infertility suggests a detrimental effect of excess nitric oxide in endometrial receptivity and implantation
Keywords: recurrent miscarriage, unexplained infertility, eNOS, endometrium, Immunohistochemistry, Real-Time RT PCR
Introduction
Recurrent miscarriage (RM) is described as at least three consecutive spontaneous pregnancy losses in the first trimester and compromises 3% of couples (Regan and Rai 2000; Taylor 2003). Genetic and developmental abnormalities are the major causes of RM, however immune, endocrine, and endometrial anatomical or non-anatomical factors as well as thrombophilias can lead to RM. In about 50% of cases, the cause of miscarriage remains unknown (Li et al. 2002a; Li et al. 2002b). Unexplained infertility (UI) affects 15% of infertile couples and is defined as when all the tests of a basic infertility evaluation including semen analysis, hysterosalpingogram, ovarian reserve testing, pelvic ultrasound, and possibly laparoscopic evaluation of the pelvis are within normal limits (Hatasaka 2011; Smith et al. 2003). Recent data has shown that UI and RM are distinctly different diagnoses and UI is not due to recurrent pre-clinical pregnancy loss (Koot et al. 2011), although these two conditions might share common endometrial function defects.
Nitric oxide (NO) is a vasodilator synthesized from L-arginine through the action of nitric oxide synthase (NOS). The three isoforms of NOS which catalyze the formation of NO are expressed in the human endometrium although eNOS is the predominant form (Khorram et al. 1999). The endometrial expression of eNOS is cyclic with peak expression during the window of implantation in humans (Khorram et al. 1999; Ota et al. 1998), and rodents (Purcell et al. 1999). Both estrogen and progesterone (Han et al. 2005; Khorram and Han 2009; Zervou et al. 1999) regulate the expression of eNOS in the human endometrium (Khorram et al. 1999). Nitric oxide by virtue of its properties as a potent vasodilator (Palmer et al. 1987), a myometrial smooth muscle relaxant (Buxton 2004; Norman et al. 1997), and its participation in signal transduction pathways (Thomas et al. 2008) might play a significant role in establishment and maintenance of pregnancy. By virtue of these properties of NO we postulated that aberrant endometrial expression of eNOS as in endometriosis (Dong et al. 2001; Khorram and Lessey 2002; Ota et al. 1998; Wu et al. 2003) and adenomyosis (Ota et al. 1998) could occur in patients with UI and RM. Since oxidative stress plays an important role at least in idiopathic recurrent pregnancy loss (Gupta et al. 2007), and NO in high concentrations can induce nitrosative stress (Agarwal et al. 2008) we hypothesized that increased endometrial eNOS expression and thereby NO generation in patients with UI and RM could impair endometrial function by either inducing cellular apoptosis (Wang et al. 2010), or through nitrosylation of key endometrial proteins (Gu et al. 2010; Weiner et al. 2009) impair their physiological function.
Materials and Methods
Sample collection and preparation of sections
The protocol for this study was approved by the Human Subjects Committee at Shahid Beheshti Medical University. Endometrial biopsies were obtained from 3 groups of women using a pipelle curette 7–9 days post ovulation as determined by serial ultrasound scans. The RM group (N=10) consisted of women with a mean age of 32.8 with a mean of 4.7 consecutive pregnancy losses. Women with secondary miscarriages or less than three miscarriages were excluded. Evaluation of RM group including karyotype analysis, antiphospholipid antibody and thrombophilia testing were all within normal range. Endometrial cavity as assessed by hysterosalpingogram was normal in RM patients. Women with unexplained infertility (N=10) consisted of individuals with a mean age of 29.8 years who were unable to conceive for more than 2 years with a normal basic infertility evaluation. This evaluation consisted of endocrine tests (TSH, cycle day 3 FSH and estradiol levels, prolactin, progesterone levels greater than 10ng/ml in mid luteal phase); anatomical tests (hysterosalpingography and pelvic ultrasonography), and semen analysis (WHO criteria). The control group (N=10) consisted of women with a mean age of 36.1 years who presented for tubal sterilization. Women in this group had normal menstrual cycles (26–33 days), had a mean parity of 1.4 and had no prior history of pregnancy losses and no prior use of assisted reproductive techniques for conception. Endometrial biopsy specimens were divided into 3 portions; one piece was placed in 4% paraformaldhyde tissue fixative for 24h and then switched to 70% ethanol for later processing. Another piece was placed in RNA Later preservative and stored at −80°C, and one section was fixed for histological dating of the endometrium using the criteria of Noyes et al. (Noyes et al. 1975). Human placental tissue was used as a positive control (Bhuiyan et al. 2006).
Quantitative Immunohistochemistry
Endometrial specimens were cut into 6μm sections using a Cryocut and placed on poly l-lysin coated slides and stored at −70°C for immunostaining. A monoclonal mouse antihuman antibody [6H2] (ABcam Company, UK, Cat.NO:91205) was used to detect the intensity and distribution of eNOS immunostaining using standard immunohistochemical protocol. Briefly after rinsing the slides with buffer (0.1 M of phosphate-buffered saline with a pH of 7.4), endogenous peroxidases were quenched by incubation in 0.3% H2O2 in methanol for 15 minutes. Repeated rinses with 0.05% bovine serum albumin in phosphate-buffered saline were performed followed by antigen retrieval using trypsin. 1.5% normal goat serum (DAKO Co., Denmark) was then added to the slides in humidified chambers for 20 minutes at room temperature to prevent nonspecific binding of antibody. The primary antibody against eNOS (1:100) was added to slides and incubated at 37°C for 1h. Slides were then rinsed three times with wash buffer, followed by incubation of sections with the secondary antibody, a rabbit antimouse IgG H&L (ABcam Company, Cat.NO: ab6728, UK) diluted 1:1000 in PBS. Incubation with the secondary antibody was performed for 1h at 37°C in an incubator. Slides were then exposed to 3, 3-diaminobenzidine in H2O2 (DAKO co, Denmark) was for 15 minutes. Thereafter, the sections were counterstained with hematoxylin and mounted. In case of negative controls similar method was used but phosphate-buffered saline replaced the primary antibody. Human full term placental tissue served as an external positive control. Staining intensity of sections was determined by Image Pro Plus software by a blinded reviewer (TN) using previously described methods (Khorram et al. 2007). 6 different areas of the sections were analyzed at a magnification of 40x and the mean was used for statistical analysis. The results were expressed as % integrated Optical Density (%IOD).
Real-Time Reverse Transcriptase–Polymerase Chain Reaction
RNA was isolated using the High Pure RNA Isolation Kit (Roche applied science). Ribonucleic acid was DNase treated and quantitated by measurement of absorbance in a NanoDrop spectrophotometer. One microgram of total RNA was reverse transcribed into single stranded complementary DNA (cDNA) with use of the Omniscript Reverse Transcription kit (GeneON, Germany) at 37°C for 60 minutes in a total volume of 20 uL. The polymerase chain reaction (PCR) mix consisted of 1 mL of 10-fold diluted cDNA, qPCR MasterMix Plus for SYBR green I reagent (GeneON, Germany) and optimized forward and reverse gene-specific primers (300 nmol/L each). Real-time PCR reactions in triplicate were run in 96-well plates with use of an Mx3000P real-time PCR system (Stratagene, Santa Clara, CA). Reactions were started by activation of DNA polymerase at 95°C for 10 minutes followed by 40 PCR cycles of denaturing at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute. The internal control used was human HPRT (de Kok et al. 2005). Data was analyzed to select a threshold level of fluorescence that was in the linear phase of the PCR product accumulation (the threshold cycle [CT]) for that reaction. The CT value for HPRT was subtracted from the CT value of eNOS gene to obtain a delta CT (DCT) value. The relative fold change for each gene was calculated with use of the ΔΔCT method (Livak and Schmittgen 2001).
Data analysis
Results were analyzed by ANOVA comparing %IOD for the imunohistochemical data and fold change in case of Real time RT-PCR using SPSS software. Post hoc analysis was done using the Student- Newman- Keuls test. P<.05 was considered statistically significant.
Results
The demographics of the three groups of women studied are shown in Table 1. Women in the UI group were significantly younger than the controls. There were no differences in BMI among the three groups. Four subjects in the control group, and two in the RM and UI groups were light smokers (<3 cigarettes/day). None of the subjects had chronic medical illnesses. Laparoscopic evaluation in all three groups showed a normal pelvis.
Table 1.
Shows the demographics of the study populations. Values are mean±SEM.
| Age | BMI | Live Birth | Miscarriage | |
|---|---|---|---|---|
| Control | 36.1± 1.1 | 26.1± 1.6 | 1.4± 0.2 | 0 |
| Recurrent Miscarriage | 32.8± 1.9 | 23.9± 1 | 0 | 4.7± 0.7 |
| Unexplained Infertility | 29.8±0.8 | 25± 1 | 0 | 0 |
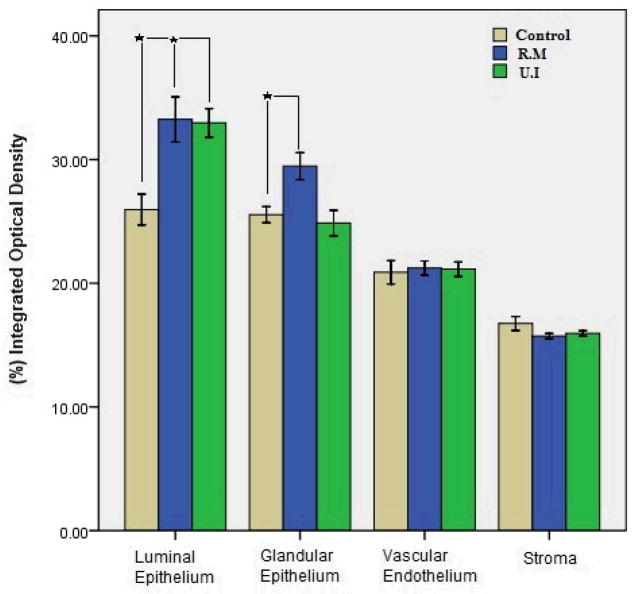
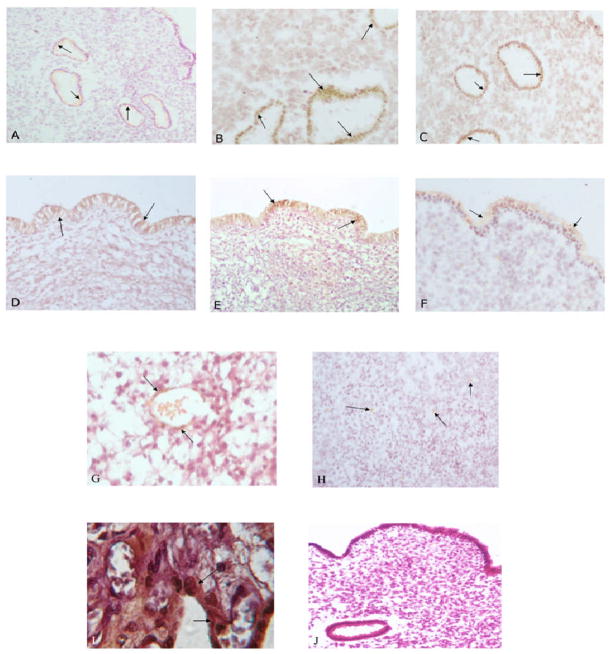
As previously demonstrated eNOS was expressed in all layers of the endometrium (Fg.1). Representative sections demonstrating eNOS protein staining in glandular (panels A–C) and luminal epithelium (panels D–F) (most intense staining) for the three studied groups are shown in this figure. Weak staining of eNOS was detected in the stromal layer (panel H), and vascular endothelium (panel G). eNOS expression was significantly higher in glandular epithelium of patients with RM (panel B) and UI (panel C) compared with control (panel A). A similar pattern was found in the luminal epithelium with greater expression of eNOS in patients with RM (panel E) and UI (panel F) compared with controls (Panel D). Negative and positive controls for eNOS are shown in panels I and J respectively. A summary of image analysis for eNOS expression in different layers of the endometrium are shown in Fig. 2. In women with recurrent miscarriage glandular (P=0.004) and luminal epithelial (P=0.002) expression of eNOS are greater compared with controls, whereas stromal (P=0.14) and vascular endothelial expression of eNOS was not significantly different in RM and UI women as compared with controls. In women with unexplained infertility the expression of eNOS was only higher in luminal epithelium compared with controls with no significant differences in other layers.
Figure 2.
Bar plot summarizing eNOS protein quantification by image analysis in 4 compartments of the endometrium in control, recurrent miscarriage (RM) and unexplained infertility (UI) groups. * Statistical Significance.
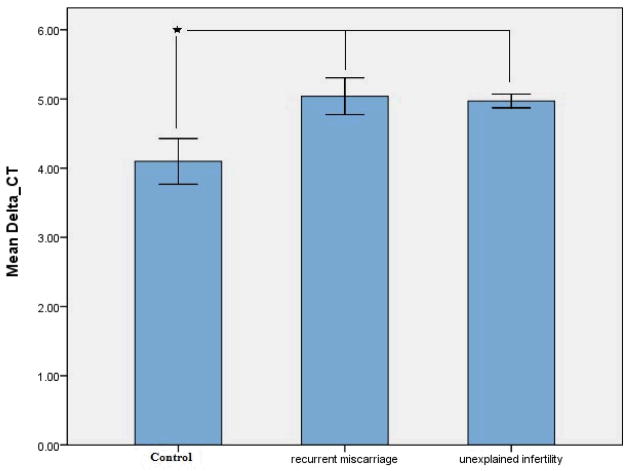
The expression of eNOS mRNA in the endometrium of women with RM and UI was significantly (P<0.05) higher compared with controls (Fig. 3) confirming the protein data.
Figure 3.
Shows eNOS mRNA levels in whole endometrial tissue homogenates in control, recurrent miscarriage and unexplained infertility groups.
Discussion
Our data confirms our prior reports on endometrial eNOS immunolocalization (Khorram et al. 1999), and demonstrates a differential site specific alteration in expression of eNOS protein in women with UI and RM compared with controls during the window of implantation. The most prominent changes were found in the luminal epithelium with greater expression of eNOS protein in both the RM and UI groups in this area. The expression of eNOS mRNA in ednometirum of women with UI and RM was significantly higher compared with controls thus confirming the protein data. The luminal eNOS protein changes in both RM and UI groups support the importance of this enzyme for implantation and its dysregulation as a possible cause of implantation failures in these patients.
The significance of NO in the implantation process has been demonstrated in animal studies in which pharmacological blockers of NOS impair implantation. Novaro et al. demonstrated that that the expression of NOS and PGE and PGF increase 1 day before implantation and suppression of NOS by L-NAME decreased production of PGE and PGF2 on the day of implantation in rats (Novaro et al. 1996). Biswas et al (Biswas et al. 1998) injected L-NAME into the rat uterine horn and demonstrated an inhibition of implantation, and similar results were obtained by Duran-Reyes et al using a different NOS inhibitor (Duran-Reyes et al. 1999). In contrast to these studies knockout mice for various isoforms of NOS do not show reduced litter size (Huang et al. 1993; Huang et al. 1995; MacMicking et al. 1995), indicating the importance of multiple pathways and redundancy in the implantation process. Since implantation failure secondary to endometrial factors may be common to UI (Koot et al. 2011) and RM (Li et al. 2002b) we sought to determine if aberrant endometrial expression of eNOS could be associated with these two conditions. The common finding of luminal increase in the expression of eNOS protein in both RM and UI patients suggest that luminal eNOS is important in the pathogenesis of these disorders. Excess NO could impair implantation through several mechanisms. NO has been shown to induce endometrial epithelial apoptosis (Castro et al. 2002; Johnson et al. 2004; Li et al. 2001), and increased eNOS expression and therefore localized excess NO production at the luminal surface could induce epithelial apoptosis and implantation failure. Vatansever et al also reported increased eNOS immunoreactivity in the endometrium of unexplained infertility patients, although this was associated with lower number of apoptotic cells (Vatansever et al. 2005). A second mechanism by which NO might impair implantation is through localized nitrosative stress. Nitric oxide by virtue of its unpaired electron is a highly reactive free radical which in excess can damage protein, carbohydrates, nucleotides and lipids (Agarwal et al. 2008). Based on our data we propose that excess eNOS expression in luminal epithelium of patients with RM and UI can create local oxidative stress which could impair implantation, similar to other inflammatory gynecologic conditions such as endometriosis (Dong et al. 2001; Khorram and Lessey 2002; Ota et al. 1998; Wu et al. 2003), adenomyosis (Ota et al. 1998), and adhesions (Saed and Diamond 2004). Exogenous factors such as cigarette smoking which has been associated with recurrent miscarriages (Cramer and Wise 2000) can also induce nitrosative stress through direct endometrial cell stimulation of eNOS expression, an effect which can be blocked by anti-oxidants such as ascorbic acid (Khorram et al. 2010). Similarly ascorbate has recently been shown to activate eNOS activity by rapid modulation of its phosphorylation status (Ladurner et al., 2012).
Several studies have attempted to find a genetic link for RM, and in so doing have examined polymorphmism of eNOS gene in different ethnic groups A recent meta- analysis of these studies showed a significant association in eNOS (Glu298Asp) polymorphism and RM (Su et al. 2011). These genetic abnormalities could lead to reduced NO production and impaired endometrial function. Our data did not show any differences in endothelial microvascular eNOS levels in RM and UI patients, suggesting that localized endometrial blood flow mediated by the NO pathway during the implantation window is not a significant factor in the pathogenesis of these disorders, and other blood flow regulating factors not examined in this study may be of greater importance. Although weak expression of eNOS in vascular endothelium as seen in our endometrial samples could be a factor which can lead to infertility and miscarriage, these expression levels were not significantly different compared to the control group. This suggests that that dysregulated expression of eNOS in non-endothelial sites plays a more a significant role in the pathophysiology of recurrent miscarriages and unexplained infertility.
In conclusion our data demonstrates an over expression of eNOS protein and mRNA in the endometrium of RM and UI. This aberrant pattern of eNOS protein and mRNA expression is similar to other inflammatory gynecologic conditions such as endometriosis and adenomyosis. Although eNOS expression in some amounts is essential for implantation, excess eNOS expression and therefore excess generation of NO in the endometrium of patients of with RM and UI is deleterious and could induce nitrosative stress which could lead to implantation failures or failure of early pregnancy maintenance.
Figure 1.
Shows eNOS staining in different endometrial compartments of the study population. Panel A shows immunostaining of eNOS in glandular epithelium of a control subject, RM patient (panel B), UI patient (panel C). Panel D shows eNOS staining in luminal epithelium of a control subject (panel D), RM patient (panel E), and UI patient (panel F). Panel G shows eNOS staining in vascular endothelium, and stroma (Panel H) of a control subject. Panel I is the negative control and panel J is positive control showing eNOS staining in a human placental section. Magnification is 40x.
Acknowledgments
The authors thank the secretarial assistance of Ms. Jeannie Park.
Supported by NIH RO3 HD 41409-01 (Omid Khorram) and Infertility and Reproductive Health Research Center grant (Marefat Ghaffari Novin).
References
- Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MB, Murad F, Fant ME. The placental cholinergic system: localization to the cytotrophoblast and modulation of nitric oxide. Cell Commun Signal. 2006;4:4. doi: 10.1186/1478-811X-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Kabir SN, Pal AK. The role of nitric oxide in the process of implantation in rats. J Reprod Fertil. 1998;114:157–161. doi: 10.1530/jrf.0.1140157. [DOI] [PubMed] [Google Scholar]
- Buxton IL. Regulation of uterine function: a biochemical conundrum in the regulation of smooth muscle relaxation. Mol Pharmacol. 2004;65:1051–1059. doi: 10.1124/mol.65.5.1051. [DOI] [PubMed] [Google Scholar]
- Castro A, Johnson MC, Anido M, Cortinez A, Gabler F, Vega M. Role of nitric oxide and bcl-2 family genes in the regulation of human endometrial apoptosis. Fertil Steril. 2002;78:587–595. doi: 10.1016/s0015-0282(02)03304-6. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wise LA. The epidemiology of recurrent pregnancy loss. Semin Reprod Med. 2000;18:331–339. doi: 10.1055/s-2000-13722. [DOI] [PubMed] [Google Scholar]
- de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–159. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
- Dong M, Shi Y, Cheng Q, Hao M. Increased nitric oxide in peritoneal fluid from women with idiopathic infertility and endometriosis. J Reprod Med. 2001;46:887–891. [PubMed] [Google Scholar]
- Duran-Reyes G, Gomez-Melendez MR, Morali-de la Brena G, Mercado-Pichardo E, Medina-Navarro R, Hicks-Gomez JJ. Nitric oxide synthesis inhibition suppresses implantation and decreases cGMP concentration and protein peroxidation. Life Sci. 1999;65:2259–2268. doi: 10.1016/s0024-3205(99)00491-9. [DOI] [PubMed] [Google Scholar]
- Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol. 2010;41:55–72. doi: 10.1007/s12035-010-8113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv. 2007;62:335–347. doi: 10.1097/01.ogx.0000261644.89300.df. [DOI] [PubMed] [Google Scholar]
- Han G, Magee T, Khorram O. Regulation of nitric oxide synthase isoforms by estrogen in the human endometrium. Fertil Steril. 2005;84(Suppl 2):1220–1227. doi: 10.1016/j.fertnstert.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hatasaka H. New perspectives for unexplained infertility. Clin Obstet Gynecol. 2011;54:727–733. doi: 10.1097/GRF.0b013e3182353e54. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Johnson MC, Maliqueo M, Boric MA, Villavicencio A, Vantman D, Vega M. Differential in vitro actions of nitric oxide on human endometrial cell survival. Fertil Steril. 2004;81:176–184. doi: 10.1016/j.fertnstert.2003.05.018. [DOI] [PubMed] [Google Scholar]
- Khorram O, Garthwaite M, Magness RR. Endometrial and myometrial expression of nitric oxide synthase isoforms in pre- and postmenopausal women. J Clin Endocrinol Metab. 1999;84:2226–2232. doi: 10.1210/jcem.84.6.5759. [DOI] [PubMed] [Google Scholar]
- Khorram O, Han G. Influence of progesterone on endometrial nitric oxide synthase expression. Fertil Steril. 2009;91:2157–2162. doi: 10.1016/j.fertnstert.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorram O, Han G, Magee T. Cigarette smoke inhibits endometrial epithelial cell proliferation through a nitric oxide-mediated pathway. Fertil Steril. 2010;93:257–263. doi: 10.1016/j.fertnstert.2008.09.074. [DOI] [PubMed] [Google Scholar]
- Khorram O, Khorram N, Momeni M, Han G, Halem J, Desai M, Ross MG. Maternal undernutrition inhibits angiogenesis in the offspring: a potential mechanism of programmed hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R745–R753. doi: 10.1152/ajpregu.00131.2007. [DOI] [PubMed] [Google Scholar]
- Khorram O, Lessey BA. Alterations in expression of endometrial endothelial nitric oxide synthase and alpha(v)beta(3) integrin in women with endometriosis. Fertil Steril. 2002;78:860–864. doi: 10.1016/s0015-0282(02)03347-2. [DOI] [PubMed] [Google Scholar]
- Koot YE, Boomsma CM, Eijkemans MJ, Lentjes EG, Macklon NS. Recurrent pre-clinical pregnancy loss is unlikely to be a ‘cause’ of unexplained infertility. Hum Reprod. 2011;26:2636–2641. doi: 10.1093/humrep/der217. [DOI] [PubMed] [Google Scholar]
- Ladurner A, Schmitt CA, Schrachner D, Atanasov AG, Werner ER, Drisch VM, Heiss EH. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phophorylation status. Free Radic Biol Med. 2012;52:2082–2090. doi: 10.1016/j.freeradbiomed.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Chang SP, Yuan CC, Chao HT, Ng HT, Sung YJ. Nitric oxide induces extensive apoptosis in endometrial epithelial cells in the presence of progesterone: involvement of mitogen-activated protein kinase pathways. Mol Hum Reprod. 2001;7:755–763. doi: 10.1093/molehr/7.8.755. [DOI] [PubMed] [Google Scholar]
- Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002a;8:463–481. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- Li TC, Tuckerman EM, Laird SM. Endometrial factors in recurrent miscarriage. Hum Reprod Update. 2002b;8:43–52. doi: 10.1093/humupd/8.1.43. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Norman JE, Ward LM, Martin W, Cameron AD, McGrath JC, Greer IA, Cameron IT. Effects of cGMP and the nitric oxide donors glyceryl trinitrate and sodium nitroprusside on contractions in vitro of isolated myometrial tissue from pregnant women. J Reprod Fertil. 1997;110:249–254. doi: 10.1530/jrf.0.1100249. [DOI] [PubMed] [Google Scholar]
- Novaro V, Rettori V, Gonzalez ET, Jawerbaum A, Faletti A, Canteros G, de Gimeno MA. Interaction between uterine PGE and PGF2 alpha production and the nitridergic system during embryonic implantation in the rat. Prostaglandins. 1996;51:363–376. doi: 10.1016/0090-6980(96)00043-3. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Hatazawa J, Tanaka T. Endothelial nitric oxide synthase in the endometrium during the menstrual cycle in patients with endometriosis and adenomyosis. Fertil Steril. 1998;69:303–308. doi: 10.1016/s0015-0282(97)00478-0. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Purcell TL, Given R, Chwalisz K, Garfield RE. Nitric oxide synthase distribution during implantation in the mouse. Mol Hum Reprod. 1999;5:467–475. doi: 10.1093/molehr/5.5.467. [DOI] [PubMed] [Google Scholar]
- Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:839–854. doi: 10.1053/beog.2000.0123. [DOI] [PubMed] [Google Scholar]
- Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11:307–314. doi: 10.1016/s1074-3804(05)60041-2. [DOI] [PubMed] [Google Scholar]
- Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003;290:1767–1770. doi: 10.1001/jama.290.13.1767. [DOI] [PubMed] [Google Scholar]
- Su MT, Lin SH, Chen YC. Genetic association studies of angiogenesis- and vasoconstriction-related genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:803–812. doi: 10.1093/humupd/dmr027. [DOI] [PubMed] [Google Scholar]
- Taylor A. ABC of subfertility: extent of the problem. BMJ. 2003;327:434–436. doi: 10.1136/bmj.327.7412.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever HS, Lacin S, Ozbilgin MK. Changed Bcl:Bax ratio in endometrium of patients with unexplained infertility. Acta Histochem. 2005;107:345–355. doi: 10.1016/j.acthis.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen C, Loake GJ, Chu C. Nitric oxide: promoter or suppressor of programmed cell death? Protein Cell. 2010;1:133–142. doi: 10.1007/s13238-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D, Khankin EV, Levy Y, Reznick AZ. Effects of cigarette smoke borne reactive nitrogen species on salivary alpha-amylase activity and protein modifications. J Physiol Pharmacol. 2009;60(Suppl 5):127–132. [PubMed] [Google Scholar]
- World Health Organization. WHOLaboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction. 4. Cambridge: Cambridge University Press; 1999. p. 128. [Google Scholar]
- Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003;18:2668–2671. doi: 10.1093/humrep/deg484. [DOI] [PubMed] [Google Scholar]
- Zervou S, Klentzeris LD, Old RW. Nitric oxide synthase expression and steroid regulation in the uterus of women with menorrhagia. Mol Hum Reprod. 1999;5:1048–1054. doi: 10.1093/molehr/5.11.1048. [DOI] [PubMed] [Google Scholar]