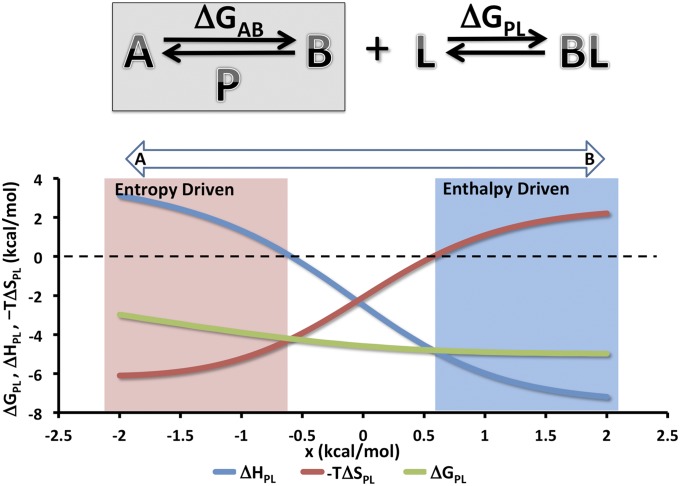
Fig. 3.
Two-state model system illustrating EET. (Upper) The protein P has two states A and B with a free energy difference of ΔGAB. By construction, state B is higher in entropy and lower in enthalpy than A. Ligand L binds only state B and hence, drives P from a two- to one-state system. The overall binding free energy is ΔGPL. (Lower) Overall thermodynamics of ligand–protein binding as a function of a small free energy bias, x, added to ΔGAB to shift the preferred unbound state of P from state A (x < 0) to state B (x > 0). Here, ΔHBL = −7.5, −TΔSBL = 2.5, ΔHAB = 10.0, −TΔSAB = −10.0, and RT = 0.6, where ΔHBL and −TΔSBL correspond to the enthalpy and entropy of the ligand L binding to state B. All quantities are in kilocalories per mole.