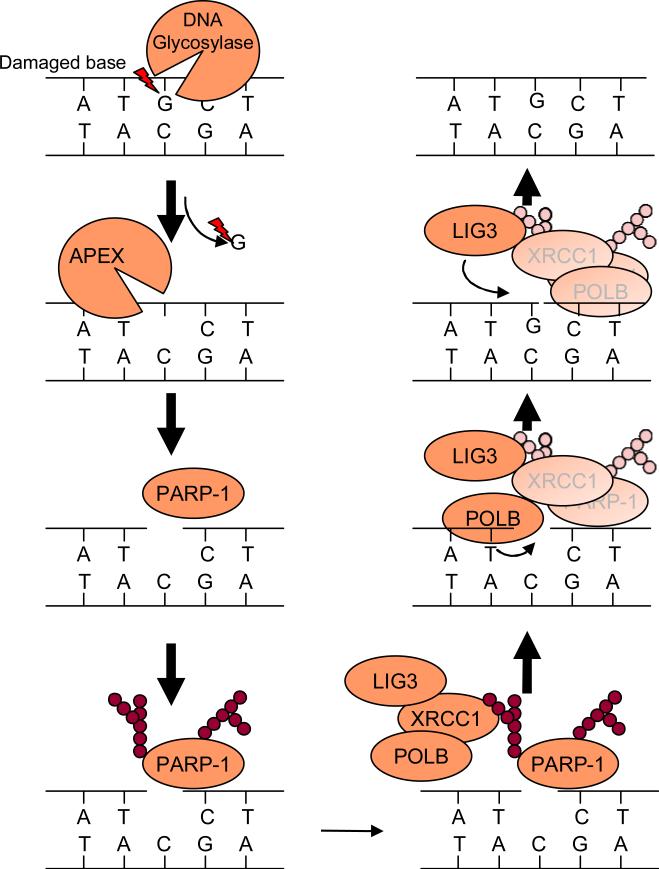
Figure 5.
The involvement of PARP-1 in base excision repair (BER). Damaged bases are recognized and cleaved by appropriate DNA glycosidase. The cleavage of an N-glycosidic bond by DNA glycosidase results in generation of apurinic or apyrmidinic site (AP site). DNA AP endonuclease or DNA AP lyase cleave the DNA backbone to create a single-strand DNA nick. PARP-1 recognizes the DNA nick as a single-strand break. The DNA binding activates PARP-1 to poly ADP-ribosylate target proteins, such as histone and PARP-1 itself. XRCC1, an important scaffold protein for BER, is recruited to where PARP-1 facilitates PAR since XRCC1 has a high affinity for PAR. XRCC1 binds to and recruits DNA polymerase beta (POLB) and DNA ligase III (LIG3) to the DNA nick. Then POLB fills the GAP and LIG3 ligases.