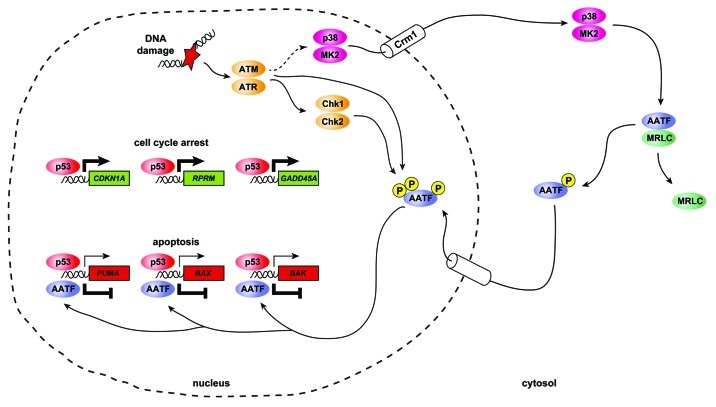
Figure 1. AATF acts as a phosphorylation-dependent molecular switch to dictate the functional outcome of p53 activation in response to genotoxic stress. Depicted is a simplified schematic overview of the regulatory imposed on the p53 response through the p38MAPK/MK2/AATF signal transduction cascade. Following genotoxic stress, the canonical DNA damage response kinases ATM and ATR are activated. Through yet uncharacterized mechanisms, ATM and ATR mediate activation of p38MAPK ultimately leading to MK2 activation. Active p38MAPK/MK2 complexes subsequently translocate from the nucleus into the cytoplasm in a Crm1-dependent process. Once in the cytosolic subcellular compartment, MK2 phosphorylates AATF on Thr-366, leading to a disruption of AATF:MRLC complexes and subsequent nuclear translocation of AATF. In addition to MK2-mediated phosphorylation on Thr-366, AATF is also directly phosphorylated by the canonical DDR kinases ATM/ATR, Chk1 and Chk2. These phosphorylation events likely occur in the nucleus. Nuclear AATF specifically engages the promoter regions of the pro-apoptotic p53 target genes PUMA, BAX and BAK leading to transcriptional repression of these genes. In contrast, AATF does not appear to bind to the promoters of the cell cycle-regulating p53 target genes CDKN1A, RPRM or GADD45A. Thus, the overall outcome of nuclear AATF activity is a repression of p53-driven apoptosis and a promotion of p53-dependent cell cycle checkpoints.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.