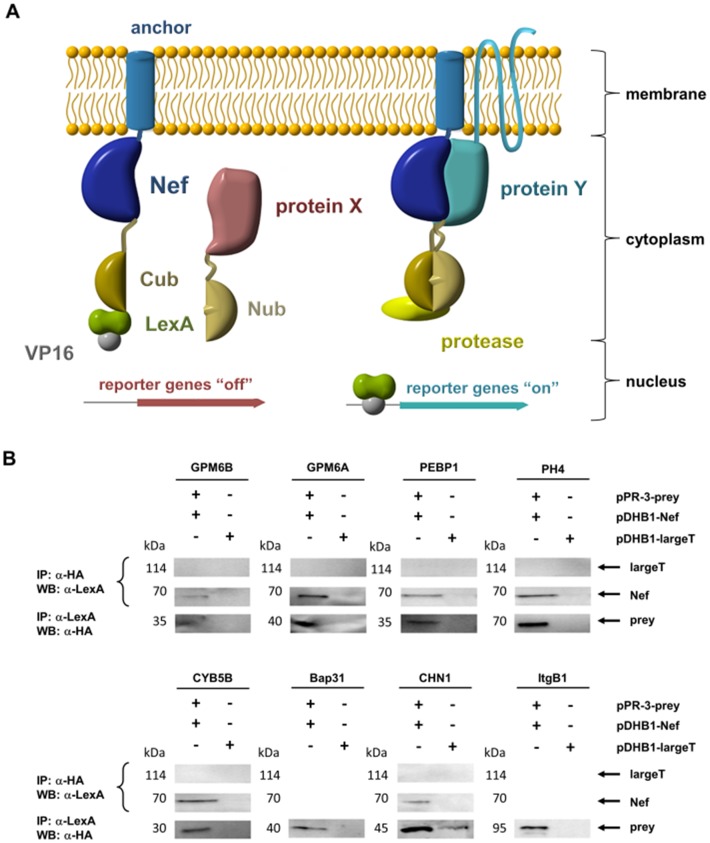
Figure 1. Schematic of the Y2H screen and coimmunoprecipitation of selected preys show interaction with Nef.
A. Schematic presentation of the split-ubiquitin Y2H screen with membrane-anchored Nef as bait. Wild type Nef myristoylation is replaced by the Ost4p transmembrane anchor and amino acids 39–76 of yeast ubiquitin (Cub) linked to the LexA-VP16 transcription factor carboxy-terminally to Nef. A human adult brain cDNA library (Dualsystems Biotech AG) cloned in pPR3-N expressing the cDNAs as fusions carboxy-terminally of amino acids 1–38 of yeast ubiquitin (Nub) and an HA-tag. Upon binding of the bait and prey both parts of the ubiquitin come together and are cleaved by the protease to activate reporter genes. If no interaction with the Nef bait protein is possible, the ubiquitin subunits stay apart and no reporter genes in the nucleus are turned on. B. Coimmunoprecipitation (CoIP) of Nef and preys. Yeast cell lysate proteins from different transfected cultures as well as a non-transfected control (NMY51) as indicated at the top were immunoprecipitated (IP) either with anti-HA or anti-LexA antibodies. For negative controls, cells were alternatively transfected with a bait expression vector coding for the SV40 large T antigen (largeT) instead of Nef. The resulting immunoprecipitates were electrophoretically separated, blotted (WB) on a PVDF-membrane as indicated at the right and detected with anti-Myc or anti-HA antibodies. The approximate molecular weights of the proteins are shown. Two additional experiments gave similar results.