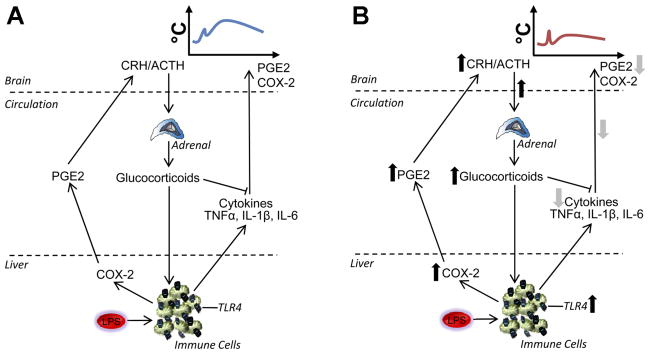
Fig. 3.
Mechanism for attenuated febrile responses in neonatally LPS-treated animals. A: in animals not treated with LPS as neonates, i.e., in the normal innate immune response, LPS acts on TLR4 on immune cells. An immune cascade is initiated whereby COX-2 is activated in liver macrophages (Kupffer cells and possibly other immune cells elsewhere), promoting PG (e.g., PGE2) release into the circulation to signal to the brain, contributing to the onset of fever. At the level of the immune cell, NF-κB also triggers synthesis and release of proinflammatory cytokines (e.g., TNFαand IL-1β or -6), which activate the brain, stimulating COX-2 to catalyze further production of PGE2. In addition to contributing to the onset of fever, PG cause an initial ACTH release from the anterior pituitary and glucocorticoid release from the adrenal cortex. Glucocorticoids have anti-inflammatory actions to limit peripheral cytokine synthesis. B: in animals treated with LPS as neonates, there is a constitutive upregulation of TLR4 on immune cells in the spleen and liver as well as constitutive upregulation of COX-2. In these animals, adult LPS causes a rapid enhanced rise in plasma PGE2 that results in an early glucocorticoid surge that acts on the immune cells to suppress cytokine production, thereby resulting in a reduced febrile response.