Abstract
The purpose of this review is to provide a comprehensive approach for assessing the upper extremity (UE) after stroke. First, common upper extremity impairments and how to assess them are briefly discussed. While multiple UE impairments are typically present after stroke, the severity of one impairment, paresis, is the primary determinant of UE functional loss. Second, UE function is operationally defined and a number of clinical measures are discussed. It is important to consider how impairment and loss of function affect UE activity outside of the clinical environment. Thus, this review also identifies accelerometry as an objective method for assessing UE activity in daily life. Finally, the role that each of these levels of assessment should play in clinical decision making is discussed in order to optimize the provision of stroke rehabilitation services.
ASSESSMENT OF UPPER EXTREMITY IMPAIRMENTS POST STROKE
Common upper extremity (UE) impairments after stroke include: paresis, loss of fractionated movement, abnormal muscle tone and/or changes in somatosensation. These impairments are a result of direct damage to the primary motor cortex, the primary somatosensory cortex, secondary sensorimotor cortical areas, subcortical structures, and/or the corticospinal tract. The evaluation determines the presence and severity of each impairment and how the impairments are contributing to loss of movement and function. Systematic, routine measurement of impairments are critical for clinical decision making 1-3. Each impairment is discussed below and common methods used for assessment are provided in Table 1.
Table 1.
Assessment of common upper extremity impairments after stroke
| Impairment | Assessment | Time to administer |
Description | Reliability | Details |
|---|---|---|---|---|---|
| Paresis | Motricity Index 119, 120 |
3 min | Manual Muscle Test Score is given for shoulder abduction, elbow flexion and pinch grip. Together these scores are converted to a total force production score for each UE ranging from 0 (no strength) to 100 (full strength) |
Intrarater =no established studies Interrater ?=0.88 |
Paresis after stroke similarly affects movement at each segment. This means that one needs to test only a UE few segments. The Motricity Index is quick and provides a total force score for the entire UE. |
| Grip Strength Pinch Strength 121, 122 |
2-3 min | Hand-Held Dynamometer to assess kilograms or pounds of force. Age- and genderappropriate normative values available |
Intrarater r=0.80 Interater r=0.97 |
Measurement of handgrip strength has been shown to predict motor performance and functional independence 64, 65 |
|
| Fractionation of Movement |
Observation of fractionated movement, paresis assessment |
Observed during paresis assessment; 5-6 min |
Presence or absence of movement fractionation observed; note any substitutions or associated reactions |
N/A | As part of the paresis assessment, fractionation of movement can be assessed. |
| Muscle Tone | Modified Ashworth Scale 31 |
< 5 min | Six point scale from 0 (no increase in muscle tone) to 4 (affected part is rigid) |
Intrarater =no established studies Interrater tau=0.85 |
The elbow flexors are most easily and commonly assessed in the UE. |
| Somatosen -sation |
Light Touch | 1-2 min | Light touch sensation can be noted as Intact, Impaired (i.e. less feeling compared to other side), or Absent based on one light stroke to the skin of the UE |
N/A | Loss of somatosensation after stroke typically occurs across multiple modalities and across the entire limb. Light touch is the most common modality assessed. Results from a single, representative modality at 1-2 sites are an indicator that similar deficits exist in other modalities and at other locations. |
| Multiple Impairments |
Fugl-Meyer, Upper Limb Section 123,84 |
30 min | The upper limb section has 33 items including: movement observation, reflex testing, grasp testing and coordination. Three point scale from 0 (unable to perform) to 2 (able to perform) totaling 66 for the upper limb portion. |
Intrarater ICC= 0.99 Interrater ICC=0.96 |
The Fugl-Meyer provides a global assessment of UE impairment. Often a quicker measure of paresis is selected over the Fugl- Meyer to decrease testing burden 124. |
Paresis
The most common motor impairment seen after stroke is paresis4. Paresis is a decreased ability to volitionally activate motor units 5-10 and is caused by damage to the corticospinal system (the primary motor cortex, non-primary cortical motor areas, corticospinal tract). Poor or absent volitional control of motor units means that muscles and sets of muscles cannot be activated in a timely, coordinated manner nor activated with sufficient force 11-21. Clinically, paresis appears as weakness and results in slower, less accurate, and less efficient movements compared to those in neurologically-intact individuals 22, 23. A stroke will cause paresis on one side of the body, contralateral to the lesioned brain, i.e. hemiparesis. Individuals with mild paresis will have movements that appear to be normal or near normal, while those with severe paresis, or plegia, may not be able to move at all. Contrary to common perception, it has been shown that the severity of paresis is similar across all segments of the UE and is not worse at the distal segments compared to proximal ones 24, 25.
Loss of Fractionated Movement
Fractionation of movement is the ability to voluntarily move one segment independently of other segments. Like paresis, fractionated movement deficits may be present after stroke damages the corticospinal system. The corticospinal system is the primary neural substrate for the enormous repertoire of complex, skilled movements humans can perform with the upper extremities 26, 27. Stroke-induced damage results in a decreased ability to selectively activate muscles 16, 21, 28; this is the same phenomenon as the “associated reactions” and “abnormal synergies” commonly described after stroke 29. Loss of ability to fractionate movement is also not specific to distal segments but has been demonstrated across all segments of the UE 30. For example, inadvertent flexion of the shoulder, wrist, and/or fingers may occur during instructed or voluntary flexion of the elbow. Because fractionation of movement is essential for skilled UE motor control 26, a reduced ability to fractionate movement can limit function.
Abnormal Muscle Tone
Muscle tone is the resistance of muscle to passive elongation or stretch 31. There is a broad range of normal muscle tone seen in healthy individuals. Abnormal muscle tone is often separated into two major categories: hypotonicity and hypertonicity. Hypotonicity is reduced muscle tone resulting from a decreased or absent neural drive to the muscle 32. It is often seen acutely after stroke as a result of damage to the corticospinal neurons, appearing as a decreased resistance to passive movement and a decreased or absent stretch reflex response 33. Hypertonicity, also referred to as spasticity or hyperreflexia, is increased muscle tone resulting from a loss of inhibition to the spinal cord as a result of damage to the corticospinal tract. Clinically, hypertonicity can be seen as an increased resistance to passive movement (spasticity = the velocity-dependent resistance) and an increased stretch reflex response 33. In this case the limb may be harder to move and range of motion may be limited. Typically after stroke, hypotonicity is seen first, and then hypertonicity develops during the first few weeks and months.
Loss of Somatosensation
If stroke damages the ascending somatosensory pathways and/or the somatosensory cortical areas, then individuals will have a reduction or loss of somatosensation. As a consequence of this loss, the nervous system has less ability to monitor and correct movement. The clinical picture of somatosensory loss due to stroke is global, generally affecting an entire side of the body. There are numerous impairment-based measures that exist to assess impairments of somatosensory modalities (light touch, joint position sense, vibration, etc.). The modality most often tested by occupational and physical therapists is light touch 34. People post-stroke who have somatosensory loss in one modality, such as light touch, typically have somatosensory loss in other modalities, such as proprioception 35. It is therefore reasonable in people post-stroke to do a quick screen of one somatosensory modality, light touch, on only one or two places on the affected limb.
CONTRIBUTIONS OF UPPER EXTREMITY IMPAIRMENTS TO LOSS OF FUNCTION POST STROKE
Although each of the impairments listed above can occur in isolation, more often they exist in combinations. This is because the stroke-induced impairments are either caused by damage to the same neurological structures (e.g. paresis, loss of fractionated movement, and abnormal tone due to damage to the corticospinal system) or caused by damage to adjacent structures (e.g. paresis and loss of somatosensation due to damage to the primary motor cortex and its neighbor, the primary somatosensory cortex). Thus, the severity of impairments is generally similar. In the UE, the severity of paresis is highly correlated with the ability to make fractionated movement 30. Similarly, the degree of spasticity, or abnormal muscle tone matches reasonably well to the severity of paresis 30. People with more severe paresis and hypertonicity have less ability to fractionate movement, and people with more mild paresis and minimal hypertonicity can make well-fractionated movements. While there are some cases of severe paresis (plegia) and hypotonicity (flaccidity), these are less commonly seen than cases with severe paresis and severe hypertonicity (spasticity).
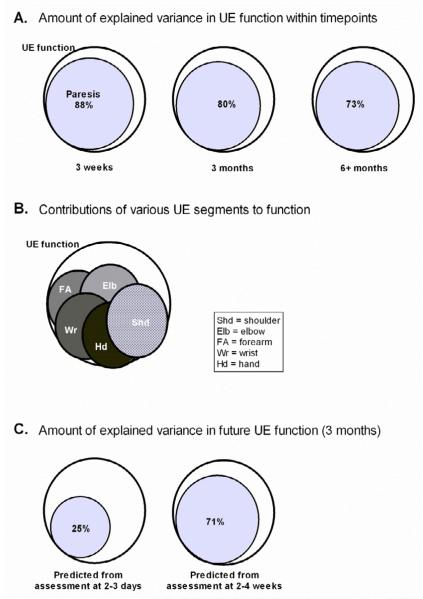
An essential issue in the assessment of the UE post stroke is how the presence of various impairments contributes to loss of UE function. Here, we use the term function to indicate the capacity to perform activities with the UE. Our laboratory has studied this issue in multiple samples over the past 6 years. Rather surprisingly, our results are remarkably consistent and show that paresis is the biggest contributor to loss of UE function post stroke 24, 25, 30, 36, 37. Figure 1 is a schematic representation of these data. Three important points are illustrated in the figure. First, at nearly all time points post stroke, the severity of paresis can explain the majority of variance in UE function (Figure 1A). While other impairments were related to function, they were not as strongly related and did not explain any additional variance, beyond that accounted for by paresis. This finding makes intuitive sense – if segments of the limb cannot move or move much, then function will be absent or poor. Second, loss of UE function stems from paresis across the limb, and not paresis at just a few segments (Figure 1B). The whole limb is needed for function 38. In order to interact with objects in the environment, the proximal segments transport and rotate the hand. This positions the hand so that the more distal segments can then contact and interact with objects. And third, paresis a few weeks, but not a few days after stroke can predict later UE function (Figure 1C). This third finding illustrates the importance of both initial severity and rate of change of severity as prognostic indicators of eventual motor function 39 (see later discussion of prognosis in this paper). A few days after stroke, measurements indicate the initial severity of paresis, but not the rate of change. By 3 weeks post-stroke, measurements reflect both. In sum, paresis is the most important impairment causing UE functional loss. It is paresis across the entire limb that leads to decreased UE function, and the severity of paresis at 3 or more weeks post-stroke that is the strongest indicator of present and eventual UE function.
Figure 1.
Schematic representation of data from multiple studies in our lab 24, 25, 30, 36-38. In each panel, the large, white circles represent the construct of UE function. The size of the smaller, filled circles represent how much the impairment (A & C, paresis) or joint (B) contributes to UE function. A: At nearly all time points post stroke, the severity of paresis can explain the majority of variance in UE function. B: Loss of UE function stems from paresis across the limb, and not paresis at just a few joints. C: Paresis a few weeks, but not a few days after stroke can predict later UE function. Other impairments tested included muscle tone, fractionation of movement, and somatosensory loss. These other impairments did not add any additional contributions to UE function in the regression models. UE = upper extremity.
ASSESSMENT OF UPPER EXTREMITY FUNCTION IN CLINIC/LABORATORY
Restoring functional use of the UE is a key goal of people who experience a stroke. Evaluation of the affected UE examines two key factors: 1) identification of the impairments limiting normal movement (discussed above), and 2) the initial level of activity limitations and participation restrictions arising from these impairments. Measurements at the activity and participation levels are important in order to address complex issues such as quality of life 40. Outcome assessments at the activity and participation levels are necessary for determining if the selected rehabilitation intervention results in changes that are important to the daily life of individuals living with stroke.
A variety of measures available for assessing UE function
Numerous measures are readily available to clinicians for the evaluation of UE function post-stroke. Many of these measures have been thoroughly evaluated for reliability and validity at multiple time points post stroke. The measures can be generally divided into two categories: 1) performance measures, where the clinician rates or times a series of UE actions that are performed by the patient, or self-report measures, where the clinician asks a series of questions about UE actions that are answered verbally by the patient or by proxy. The most frequently cited UE performance measures include the Action Research Arm Test (ARAT), Box and Blocks Test (BB), Chedoke Arm and Hand Activity Inventory (CAHAI), Jebsen-Taylor Hand Function Test (JTT), Nine-Hole Peg Test, and the Wolf Motor Function Test (WMFT). The most frequently cited self-report measures include the Stroke Impact Scale (SIS) and the Motor Activity Log (MAL). There is no consensus on which measure to choose for a particular person with stroke. While there is no single measure that encapsulates the entire range of activities performed by the UE 41, a recent review suggests that the ARAT and the BB test have the strongest clinical utility 42. Once a measure is selected, the same measure should be administered at evaluation, interim and discharge evaluations, in order to document progress or lack thereof. Tools to evaluate UE function are described briefly below; a summary of common psychometric properties and further details about each test are provided in Table 2.
Table 2.
Performance and self-report measures commonly used to assess upper extremity function after stroke
| Performance Assessments | ||||||
|---|---|---|---|---|---|---|
| Name | Time to administer |
Reliability | Relation to other measures (Concurrent Validity) |
Estimate of MCID* | Strengths | Weaknesses |
| Action Research Arm Test |
10-15 min | Intrarater r=0.99 Interrater r=0.98 Test-retest r=0.98 |
r=0.91-.94 with Fugl- Meyer; r=0.96 with Motor Assessment Scale ; r=0.87 with Motricity Index; r = 0.93 with CAHAI |
6 pts (chronic stroke); 12 points (acute stroke - dominant hand); 17 points (acute stroke - nondominant hand) |
Quick; Easily administered; Appropriate at all stages of recovery |
Not commerciallyavailable, but can be built from published instructions |
| Box & Blocks Test (BB) |
5-10 min | Intrarater ICC= no established studies Interrater ICC= 0.99 Test-retest ICC= 0.96 |
r=0.92 with Fugl- Meyer; r=0.95 with ARAT |
6 blocks with affected hand |
Quick; Easily administered |
Requires at least minimal distal volitional control |
| Chedoke Arm and Hand Activity Inventory (CAHAI) |
25 min | Intrarater ICC= no established studies Interrater ICC= 0.98 Test-retest ICC= 0.96 - .97 |
r = 0.93 with ARAT | 6.3 points | Easily administered; Shorter versions of the test exist if time is a concern; Free to use |
Takes longer than other measures that capture the same information |
| Jebson-Taylor Hand Function Test |
15-20 min | Interrater ICC= 0.82- 1.00 |
rs= 0.84-.97 with 9- Hole Peg Test; rs= 0.87-.95 with ARAT |
unknown | Standardized instructions; good measure when the ceiling is achieved on other shorter measures |
Requires at least minimal distal and proximal volitional control |
| Nine-Hole Peg Test |
10 min | Interrater/Test-retest: r= 0.68-.99 |
rs= 0.84-.97 with Jebsen-Taylor rs= 0.85-.93 with ARAT |
32.8 seconds with affected hand |
Quick; Inexpensive to purchase |
Most appropriate for higher performing individuals |
| Wolf Motor Function Test |
30 minutes | Interrater ICC= 0.85- 0.97 Test-retest ICC= 0.94- 0.99 |
rs= 0.86 (FAS) with ARAT rs= 0.89 (time) with ARAT |
1.5-2 sec (WMFT time - chronic stroke); 19 seconds (WMFT time -acute stroke); 0.2-0.4 pts (WMFT FAS) |
Standardized instructions; Appropriate at all stages of recovery |
Takes longer than other measures that capture the same information |
| Self Rating Assessments | ||||||
| Motor Activity Log |
15-20 minutes |
Test-retest ICC= 0.79- 0.82 |
rs=0 .35-.39 (QOM scale) with ARAT rs= 0.31-.32 (AOU scale) with ARAT rs= −0.26- −.33 (QOM scale) with 9-Hole Peg Test rs= −0.16- −.23 (AOU scale) with 9-Hole Peg Test rs= −0.52 (QOM scale) with BB rs= −0.37-.49 (AOU scale) with BB r=0.52-.66 (QOM scale) with accelerometry |
1.0-1.1 pts on the quality of movement scale |
Inexpensive; Tries to capture real-world abilities; Easy to administer |
Takes longer than other interviews; Relies on self ratings |
| Stroke Impact Scale (ADL and Hand Function Subscales) |
5 minutes each subscale |
Test-retest: ICC= .70-.92 |
rs= 0.57-.73 with ARAT rs= 0.61-.83 with Jebsen-Taylor rs= 0.53-.66 with 9- Hole Peg Test |
ADL/IADL = 5.9 pts Hand function = 17.8 pts |
Inexpensive; Tries to capture real- world abilities; Easy to administer |
Relies on self- ratings |
Minimal Clinically Important Difference (MCID) has been defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient’s management” 125. Note that these values are labeled as estimates because they are likely influenced by the time post-stroke and the severity of functional loss.
Performance Measures
Action Research Arm Test (ARAT)
Developed for patients with hemiparesis, the ARAT is a criterion-rated assessment of UE activity limitations 43-53. The ARAT includes 19 items divided into four subscales: grasp, grip, pinch, and gross movement. The items within each subtest are ordered based on a 4-point ordinal scale ranging from 0 to 3 where 3 represents normal performance on each item. The items are arranged in a hierarchy, permitting skipping some items if the person is unable to do an earlier item or performs an earlier item normally. A score of 57 indicates normal performance.
Box and Block Test (BB)
The Box and Block test is a quick and easy to administer assessment designed to quantify UE activity limitations by a person’s ability to grasp, transport, and release small blocks 53-56. Individuals are asked to move as many one-inch blocks across the center of the test box in one minute. Performance is determined by the number of blocks moved in one minute. Times are compared to established norms, with better performance indicated by a higher number of blocks moved.
Chedoke Arm and Hand Activity Inventory (CAHAI)
The CAHAI is a functional assessment used to determine how much the arm and hand have recovered after stroke 57-60. The original version of the assessment is comprised of 13 items that require the use of both arms. Each activity is rated on a 7-point quantitative scale ranging from 1= total assistance and the weak limb performs less than 25% of the task to 7= total independence. Higher scores indicate a higher level of functional independence. Three shortened versions of the test exist, the CAHAI-9, CAHAI-8 and CAHAI-7, if time to complete the assessment is a concern.
Jebsen-Taylor Hand Function Test
The Jebsen-Taylor Hand Function Test was developed to assess the use of the UE in everyday tasks 61. There are seven tasks that are tested: writing a sentence, card turning, lifting small objects, simulated feeding, stacking checkers, and picking up light and heavy cans. Each task is timed, and better performance is indicated by faster times. Age- and gender-based normative values on each test are available for comparison.
Nine-Hole Peg Test
The Nine-Hole Peg test is a brief measure used to quantify hand dexterity 56, 62-65. Performance is quantified as the time taken to place and then remove the pegs, one at a time. Times are compared to established norms, with better performance indicated by faster times. Individuals must have some degree of volitional hand movement for this test to be a useful tool.
Wolf Motor Function Test (WMFT)
The WMFT is comprised of 15 tasks to evaluate UE impairments and activity limitations 45, 46, 48, 66-72. Items 1-6 are timed joint-segment movements whereas items 7-15 are timed integrative functional movements. The person is given a time on each item and a Functional Ability Score (FAS) on a 6-point scale ranging from 0 = unable to complete the task to 5 = completes the task with normal movement. For the timed scored, better performance is indicated by faster times. For the Functional Ability Score (FAS), better performance is indicated by higher scores, usually represented as an average of the individual item scores.
Self-Report Measures
Motor Activity Log (MAL)
The MAL is a structured interview used to assess real-world UE activity 46, 50, 73-75. An individual is asked to rate his/her performance on how much and how well the affected UE is used during a variety of activities typically completed in daily life. Subscores can be derived from the Quality of Movement (QOM) scale and the Amount of Use (AOU) scale. Each scale is rated on a scale of 0 – 5 where 0= “the weaker arm was not used at all for that activity” on the QOM scale and “did not use my weaker arm” on the AOU scale and 5= “the ability to use the weaker arm for that activity was as good as before the stroke” on the QOM and “used my weaker arm as often as before the stroke” on the AOU scale. Scores on each scale are calculated as the mean of the scored items attempted with the affected arm. Averages closer to 5 indicate better quality of movement and more use of the affected arm/hand. There are two versions of the assessment; 1) MAL-28 and the shorter 2) MAL-14.
Stroke Impact Scale
The Stroke Impact Scale (SIS), is a stroke-specific, comprehensive health questionnaire with subscales in eight domains: Strength, Hand function, ADL/IADL, Mobility, Communication, Emotion, Memory and Thinking, & Participation/Role function 76-83. The individual uses a 5-point Likert scale to rate his/her ability on each item. Questions can be administered in a face-to-face interview format, over the telephone, or via the mail. The subscales of Hand Function and ADL/IADL are most relevant for measurement of UE function. Scores for each subscale range from 0 – 100, with normal self-reported function indicated by a score of 100.
Relationships between measures at different time points post-stroke
Scores for many of these measures are highly inter-related, and remain so across various times post stroke. For example, the ARAT and the WMFT have been shown to be strongly correlated with each other at 14 days, 1, 3, and 6 months post-stroke 84. Similarly, the ARAT, Jebsen-Taylor Test of Hand Function, 9-Hole Peg Test, and the Stroke Impact Scale – Hand Function subscale have been shown to have moderate to strong correlations at 1 month, 3 months and 6 months post-stroke 85. Relationships between these measures and others are provided in the fourth column in Table 2. The strength and consistency of these correlations suggest that the measures quantify the same underlying construct, i.e. UE function, and that any one of them would be appropriate for clinical use. The decision to use one measure vs. another can therefore be made based on additional factors.
Deciding which measures to use
Since measures of UE function are highly inter-related, there is a great deal of freedom when selecting a measure for use with a specific individual. Some therapists may use more than one measure, with one assessment intended to quickly quantify change (e.g. BB) and another measure intended to determine which UE movements (e.g. ARAT or WMFT) or which self-reported functional problems (e.g. MAL, SIS) need to be addressed during treatment. Three key questions influence the selection process. Is the necessary equipment available? Is specific training needed prior to administration? How much time does it take to administer?
Equipment
Many of the described measures are pre-made test kits that can be purchased from reputable rehabilitation suppliers. These test kits can range in price from approximately $50 up to several hundred dollars. Both the ARAT and the WMFT test kits can be built relatively economically based on descriptions in published articles or manuals available on the internet. A good source for information about the tools and what each tool looks like is http://www.medicine.mcgill.ca/strokengine-assess/, a web-site sponsored by the Canadian Stroke Network. Availability of measures will vary from facility to facility. For instance one facility may have the Jebsen-Taylor test while another facility may own the ARAT. Because these measures are similar assessments of UE function, then choosing the test that is readily available at one’s workplace is a logical basis for test selection.
Training
Often measures require that the administering clinician take part in a formalized training program in order to be certified to use the measure. Each of the performance assessments discussed in this manuscript do not require additional training in order to perform them. Some assessments, like the CAHAI provide training videos for a nominal fee (available online). For other assessments, like the ARAT, there is are published instructions available that can be followed to ensure stable performance 86. For most of the measures discussed here, carefully reading the instruction manual and practicing administration of the test will result in the measure being administered correctly.
Time
The amount of time required to administer a measure will strongly influence whether or not it is selected for use in routine clinical practice. Measures that take a shorter amount of time are more likely to be used. The second column in Table 2 provides an estimate of the time needed to administer each of the measures. Note that it usually takes longer to administer any measure when one first starts using it. As familiarity with the items and instructions increases, the time to administer the measure usually decreases. Clinical facilities often prescribe the amount of time available for an evaluation. While it is compelling to always choose the shortest measures (e.g. Box and Block or Nine-Hole-Peg tests), the longer measures often yield additional valuable information. The short measures require performance of only one action. The longer measures (e.g. ARAT and WMFT) evaluate performance on more actions. In addition to the total score on the longer measures, observing the person with stroke interact with a variety of objects during administration of the longer measures will allow the treating clinician to see exactly which components of movement (e.g. types of grasps) and how (e.g. with large objects, cannot extend fingers sufficiently) movement components are affected. These observations can facilitate treatment planning, providing information on how to structure movement practice.
ASSESSMENT OF UPPER EXTREMITY USE OUTSIDE THE CLINIC/LABORATORY
Assessment of the affected UE in the clinic or laboratory includes measures of impairments, measures of performance, and self-report questionnaires. Outside the clinic however, direct observation of impairment and performance is costly and impractical, leaving self-report measures as one of the only options. Although self-report measures are often selected due to ease of administration, they are: 1) affected by comprehension, memory recall, and motivation 87, 88; 2) unreliable when used with persons who have cognitive deficits in memory and attention, as commonly experienced by persons with stroke 89; and 3) at best are moderately correlated with direct methods of activity measurement 90. Furthermore, improvement on measures of function following rehabilitation has been shown to lack association with increased UE use at home 91. Because improvement in daily function at home is the goal of rehabilitation, it is important that objective assessment tools be used to measure UE use during real-world activity outside of the clinic. One such tool is the accelerometer.
How accelerometers measure upper extremity use
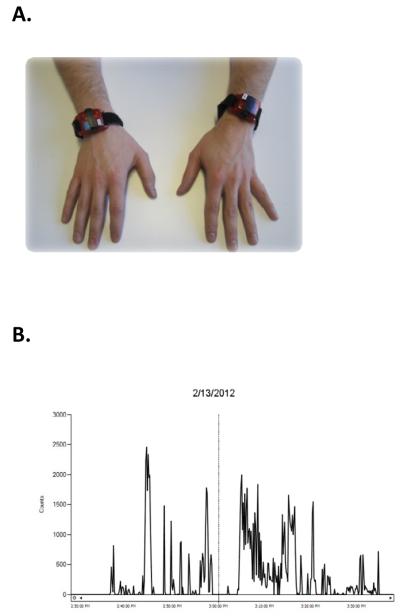
Accelerometers measure movement in terms of acceleration. Acceleration is the change in speed with respect to time, and is measured in gravitational acceleration units (g; 1 g=9.8 m/s2). This is done by converting mechanical motion into electrical signals, often via piezoelectric sensors. The sensors are contained within a device which is similar in size to a wristwatch (Figure 2A) and can be comfortably worn on the wrist 92. The electrical signal is converted to a digital signal called an activity count, quantifying how much movement occurred during a specific time period, called an epoch 93. The epoch is chosen by the clinician, and can be as short as a second or as long as a few minutes (e.g. the length of a specific activity). One way to measure UE use is to sum activity counts per epoch as a measure of intensity of UE use. Another way to measure UE use is to choose a small epoch, such as one second, and determine if movement occurred during that epoch. This is done by “filtering” activity counts: if an activity count occurred during a one-second epoch, then movement occurred during the epoch; if an activity count did not occur during the epoch, then movement did not occur during the epoch 94. In this way, epochs where the UE was used can be summed to determine the amount of UE use during a given time period, such as hours in a day. Percentage of UE use that occurred during the wearing time can also be determined. Epoch selection, filtering, and calculation of UE use can often be done with the software program that comes with the accelerometers. Figure 2B shows an example of what UE accelerometer data look like during a 11/2 hour wearing time.
Figure 2.
Accelerometry can be a useful tool to measure UE use outside of the clinic or laboratory. A: Picture of commercially-available accelerometers worn on the wrists (GT3X+ Activity Monitor, ActiGraph, Pensacola FL). The size of each accelerometer is 4.6 cm x 3.3 cm x 1.5 cm. B: An example of what UE accelerometer data looks like. The date of the recording is provided at the top, and data is shown from 2:30 pm to 4:00 pm. The data line indicates the count or how much the limb was moving during this 1.5 hour period. Moments when the line is equal to zero indicate times when the limb was not moving.
Reliability and validity of accelerometer measurement
For accelerometers to be useful, they must be reliable and valid. Studies have shown that accelerometer activity counts are consistent for the same activity at different time points when the same accelerometer unit is used, i.e. intra-rater reliability has been established 95. Activity counts are also consistent for the same activity when different accelerometer units are used, i.e. inter-rater reliability has been established 95, 96. Furthermore, strong correlations between UE accelerometer counts at separate time points indicate good test-retest reliability 97, 98.
It is well documented that accelerometry is an objective measure of UE use, i.e. construct validity has been established. Studies show 88% agreement between UE accelerometry and observed movement by an observer, as well as a very strong correlation (r = 0.93) between UE accelerometry and duration of movement. Accelerometry is also able to distinguish UE use between people with and without stroke and between use of the affected and unaffected limbs of people with stroke 99, 100. Moreover, accelerometry is sensitive to change, demonstrating increases in activity counts following therapeutic intervention 101-103.
There is also ample agreement between accelerometry and accepted, standardized measures of activity, i.e. convergent validity has been established. Moderate to strong correlations exist between UE accelerometry and electromyography during activities of daily living (r = 0.53) 104, elbow electrogoniometry during daily use (r = 0.94) 105, and standardized measures that assess impairment, function, and activity (see Table 3). Low correlations between UE accelerometry and the mobility subscale of the Stroke Impact Scale (r = 0.16 - 0.23) indicate that UE accelerometry is not associated with lower extremity activity 106, thereby demonstrating divergent validity.
Table 3.
Relationships between accelerometry measures of upper extremity use and impairment, self-report, and performance measures. Note that some of the performance measures specifically assess the upper extremity while others assess additional constructs, such as mobility and activities of daily living.
| CORRELATION | |
|---|---|
| IMPAIRMENT MEASURES | |
| Motricity Index | r=0.5* 102 |
| Fugl-Meyer Assessment—Arm Section |
r =−0.851**
126 r=0.54** 127 |
| PERFORMANCE MEASURES | |
| Action Research Arm Test | r=0.40† 99 |
| Wolf Motor Function Test | r=0.62* 99 |
| Functional Impairment Measure | r=0.67,* 99 |
| Barthel Index | ρ=0.64* 102 |
| SELF-REPORT MEASURES | |
| Stroke Impact Scale – Hand Function subscale |
r=0.61** 103 |
| Motor Activity Log – Quality Of Movement scale |
r=0.52**
98 r=0.66** 103 |
=.05,
=.01,
=.001
The utility of accelerometers for clinical practice
Accelerometers are relatively inexpensive ($100-500 per unit), commercially-available, and complement information gained from other available measures. Accelerometers can be used to measure UE use during the initial evaluation, weekly or monthly during the course of treatment, and at discharge. UE use can then be compared across time points to assess change in use. When used this way, UE use as measured by accelerometry, is an intuitive measure of recovery and return to daily activity and participation. The counts or amount of use generated by accelerometers can be appreciated by the person receiving therapy services, his/her family members, other clinicians, and third-party payers. It is relatively simple for persons receiving rehabilitation services to understand his/her UE recovery as measured by an increase in hours of UE use in a day. These numbers are more intuitive than changes of one or a few points on clinical rating scales. Direct measures of use may also motivate the individual and the family to continue efforts to incorporate gains made in therapy into everyday life. Additionally, rehabilitation clinicians will benefit from tracking change in UE use during treatment as a means of evaluating the effectiveness of the chosen interventions. If an intervention has increased the real-world use of the affected UE, these numbers are proof of the benefit. If an intervention has failed to increase the real-world use of the affected UE, then a different approach needs to be selected or therapy services need to be terminated. A quantitative measure showing increased UE use during the course of therapy services can be submitted with other documentation when requesting additional therapy services from a third-party payer. Our laboratory is in the process of collecting normative data with accelerometers; these values will be useful benchmarks for assessing the extent of improvement in individual patients.
While accelerometers provide useful information, some limitations deserve consideration. Accelerometers are electronic devices that are subject to mechanical failure and need to be checked at regular intervals. Another limitation is that accelerometer data has to be processed by a software program. Care must be exercised when choosing accelerometers and software programs to ensure that the user will be able to adjust program settings in order to report activity counts in terms of UE use. Fortunately, current available software has become substantially more user-friendly over the past 10 years, even for busy clinicians. Finally, compliance with wearing the accelerometers may be an issue for some individuals when wearing them for a long period of time, forgetting to replace accelerometers after removal for showering or hand washing, or cognitive deficits. Despite their limitations, accelerometers are practical and useful devices that allow a clinician to collect valuable, objective information that would be otherwise unavailable. They allow UE use to be measured outside of a clinical setting and to effectively measure rehabilitation outcomes in the real world.
USING ASSESSMENT RESULTS TO MAKE CLINICAL DECISIONS
Results derived from both impairment and functional assessments assist the clinician with the development of treatment plans and aid with evaluating the utility of a particular treatment. In addition to assessment results, clinical decisions depend on the prognosis for recovery of impairments and function after stroke. Epidemiological data from multiple countries show that recovery after stroke occurs along a fairly predictable time course. Most motor and functional recovery will occur within the first 3 months 107, 108. Early severity of paresis is the best predictor of eventual motor deficits and function 39, 109, 110. Those with mild deficits recover more quickly and completely, with best neurological recovery occurring within 3 – 6 weeks 108. Those with more severe deficits recover more slowly and to a much lesser extent, with best neurological recovery occurring within 13 – 15 weeks 108. For the purpose of predicting recovery of individual patients, the epidemiologic data provide the general pattern of recovery and most, but not all, patients will follow a similar time course of changes. There are three additional indicators of future outcomes that are useful to look for when trying to determine prognosis in individuals. First, the more non-motor impairments (e.g., somatosensory loss or visual field loss) that accompany the motor deficits (e.g., paresis, fractionated movement deficit), the less likely a person is to return to functional independence 111. Second, early, rapid improvements in motor impairments are an indication that a person is more likely to reach higher levels of independence 108, 112. And third, the absence of measurable grip strength or shoulder flexion at 3-4 weeks post stroke is a strong indicator that the affected upper limb will be non-functional 25, 29, 110, 113. Recovery of function typically lags recovery of neurological impairments by about 1 to 2 weeks, with the trajectory of the two recovery curves being nearly identical 108. The reason for the 1 – 2 week lag may be that as impairments improve, practice is required to capitalize on the neurological recovery and incorporate the improvements into daily function.
The combination of the individual’s assessment data, the time since their stroke, and the epidemiological data reviewed above will allow the clinician to determine: 1) whether or not more neurological recovery is expected; and 2) whether or not more functional recovery is expected. For many individuals with stroke, motor and somatosensory impairments will not substantially change, but activity limitations and participation restrictions can be lessened through a course of rehabilitation. For example, a person that is 2 years post stroke with a Motricity Index of 27 (MMT grades of 2/5 in the three candidate motions) and absent light touch sensation on the palm on their affected UE would be expected to have no or minimal changes at the impairment level. It is highly possible however, that a course of rehabilitation therapy with a focus on functional training for the individual and his/her family, may improve daily activities such as bathing and dressing and may reduce caregiver assistance needed with these activities. Determining expectations about the likelihood of neurological and functional recovery from assessment scores will facilitate: 1) setting realistic treatment goals; and 2) selecting a restorative or compensatory approach to achieve those goals.
Choosing a restorative or a compensatory approach is critical when developing the plan of care for an individual with stroke. A restorative approach is focused on returning or restoring the previously lost motor abilities and function. A compensatory approach is focused on maximizing function, often with alternate strategies, within the confines of the limited motor abilities. In managing the UE post stroke, a restorative approach would be chosen if the person had a stroke less than 3 months earlier and assessment results indicate there is voluntary, fractionated movement against gravity at several UE segments 114. In contrast, a compensatory approach would be chosen for a person with minimal or no voluntary, fractionated movement, whether early or later poststroke 114. The expectation for the restorative approach is that a course of rehabilitation will return the arm and hand to a reasonable, functional level of dexterity. The expectation for the compensatory approach is that a course of rehabilitation will educate the individual to minimize contracture development, edema, and potential hygiene problems, and possibly permit the arm and hand to be used as an assist during functional activities. In the neurological therapy world, there is an often-voiced concern that the decision to use a compensatory approach will limit a person’s eventual recovery. There is limited empirical data to support this concern. In fact, results from the VECTORS trial, a Phase II trial evaluating constraint induced movement therapy in the inpatient rehabilitation setting, suggest otherwise 115. In this study, the experimental group received focused training aimed at restoring normal movement of the affected limb while the control group received general training and could not be cued to use their affected limb or told to use it in a specific way. UE function improved to a similar degree in these two dose-matched groups, and scores were equivalent at the end of the intervention and at 3 months post stroke. An equal but less discussed concern is that the decision to use a restorative approach could waste valuable therapy time, leaving a person with stroke ill-equipped to function in daily life once the course of therapy is completed. There is no easy answer to these competing concerns. Frequent re-assessment of key impairments and activity limitations will enable the clinician to determine if the appropriate approach was chosen and if a switch to the alternate approach is needed.
Specific interventions are chosen once the approach has been determined. Interventions for the UE post-stroke are generally targeted toward improving function and not targeted at improving impairments in isolation 114, 116. The challenge to the clinician lies in selecting and structuring functional training to address the movement dysfunction (restorative and compensatory approaches) and underlying impairments (restorative approach only). For example, if the individual’s goal is to return to gardening activities, then therapy sessions and the home exercise program would be used to practice (or simulate practice of) various aspects of gardening such as grasping and using tools, digging, and moving plants. If the approach is compensation, then the specific treatment will focus on executing gardening activities safely with whatever positions (seated vs. standing), motions (primarily use unaffected hand), and assistive devices (adaptive tools) are appropriate. If the approach is restorative, then the specific treatment will focus on returning normal movement patterns while grasping and using gardening tools. While further discussion of specific interventions and their evidence of benefit is beyond the scope of this chapter, the reader is encouraged to access excellent online summaries such as the Evidence-Based Review of Stroke Rehabilitation114 (www.ebrsr.com) and the StrokeEngine (www.strokengine.ca).
Repeated administration of one or several measures is of great benefit to persons receiving stroke rehabilitation and clinicians managing their rehabilitation course. For the person with stroke, an improved score (e.g. 6 kg of grip force now compared to 3 kg two weeks ago, 41/57 on the ARAT now compared to 21/57 two weeks ago) can motivate them to continue with their long, demanding course of rehabilitation. If the scores are not improving, this information can affirm that their perception of stalled performance may be accurate. For the clinician, it is an objective, quantitative method to assess progress. Data from the measures address issues of whether or not a person is continuing to improve, whether or not the selected intervention is improving impairments and function as hoped, and whether or not continued therapy services are warranted. Clinicians will want to select a reasonable interval for re-assessment, i.e. a time in which improvements would be expected to occur. In short inpatient stays, now averaging 16-17 days in the United States 117, 118, formal re-assessment of UE impairments and activity restrictions may take place in the days before discharge. These results would then be used to educate the person with stroke and his/her family, to select appropriately graded home activities and exercises, and to provide information to the next point of care. For outpatient services, re-assessment might be most appropriate on a monthly basis, as changes in impairment and function will be occurring more slowly than they were immediately after stroke and visits will be one or a few times per week. Reassessment of functional improvement is the key factor in keeping the person, family, and physician updated on rehabilitation progress.
SUMMARY
The purpose of this review was to provide a comprehensive approach for assessing the UE after stroke. Evaluating the presence and severity of UE impairments and function after stroke is critical for understanding not only how well the UE is used in the clinic, but also how much the limb may be used during activities of daily living at home or work. Many of the assessments provided in this review are reliable and valid, with high levels of consistency (between raters and post-stroke time points) and correlation (between the assessments themselves). While knowing what constructs are being tested by each assessment is important, knowing how these assessments provide insight into the larger picture of UE function and recovery is useful in formulating goals and treatment plans for individuals living with stroke.
Acknowledgements
Partial salary support was provided by NIH R01 HD055964 (CEL, MDB, RLB), NIH T32 HD007434 (RRB) and AHA 10POST4140091 (SYS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Swinkels RA, van Peppen RP, Wittink H, Custers JW, Beurskens AJ. Current use and barriers and facilitators for implementation of standardised measures in physical therapy in the netherlands. BMC Musculoskelet Disord. 2011;12:106. doi: 10.1186/1471-2474-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potter K, Fulk GD, Salem Y, Sullivan J. Outcome measures in neurological physical therapy practice: Part i. Making sound decisions. J Neurol Phys Ther. 2011;35:57–64. doi: 10.1097/NPT.0b013e318219a51a. [DOI] [PubMed] [Google Scholar]
- 3.Jette DU, Halbert J, Iverson C, Miceli E, Shah P. Use of standardized outcome measures in physical therapist practice: Perceptions and applications. Phys Ther. 2009;89:125–135. doi: 10.2522/ptj.20080234. [DOI] [PubMed] [Google Scholar]
- 4.Sathian K, Buxbaum LJ, Cohen LG, Krakauer JW, Lang CE, Corbetta M, Fitzpatrick SM. Neurological principles and rehabilitation of action disorders: Common clinical deficits. Neurorehabil Neural Repair. 2011;25:21S–32S. doi: 10.1177/1545968311410941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:907–916. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young JL, Mayer RF. Physiological alterations of motor units in hemiplegia. J Neurol Sci. 1982;54:401–412. doi: 10.1016/0022-510x(82)90203-9. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsson F, Grimby L, Edstrom L. Motoneuron activity and muscle fibre type composition in hemiparesis. Scand J Rehabil Med. 1992;24:115–119. [PubMed] [Google Scholar]
- 8.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 9.Frontera WR, Grimby L, Larsson L. Firing rate of the lower motoneuron and contractile properties of its muscle fibers after upper motoneuron lesion in man. Muscle Nerve. 1997;20:938–947. doi: 10.1002/(sici)1097-4598(199708)20:8<938::aid-mus2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.McComas AJ, Sica RE, Upton AR, Aguilera N. Functional changes in motoneurones of hemiparetic patients. J Neurol Neurosurg Psychiatry. 1973;36:183–193. doi: 10.1136/jnnp.36.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond MC, Fitts SS, Kraft GH, Nutter PB, Trotter MJ, Robinson LM. Co-contraction in the hemiparetic forearm: Quantitative emg evaluation. Arch Phys Med Rehabil. 1988;69:348–351. [PubMed] [Google Scholar]
- 12.Gowland C, deBruin H, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Phys Ther. 1992;72:624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- 13.Fellows SJ, Kaus C, Thilmann AF. Voluntary movement at the elbow in spastic hemiparesis. Ann Neurol. 1994;36:397–407. doi: 10.1002/ana.410360311. [DOI] [PubMed] [Google Scholar]
- 14.Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: Role of inappropriate muscle coactivation. Muscle Nerve. 2001;24:673–681. doi: 10.1002/mus.1054. [DOI] [PubMed] [Google Scholar]
- 15.Bourbonnais D, Vanden Noven S. Weakness in patients with hemiparesis. Am J Occup Ther. 1989;43:313–319. doi: 10.5014/ajot.43.5.313. [DOI] [PubMed] [Google Scholar]
- 16.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 17.Sahrmann SA, Norton BJ. The relationship of voluntary movement to spasticity in the upper motor neuron syndrome. Ann Neurol. 1977;2:460–465. doi: 10.1002/ana.410020604. [DOI] [PubMed] [Google Scholar]
- 18.Dietz V, Ketelsen UP, Berger W, Quintern J. Motor unit involvement in spastic paresis. Relationship between leg muscle activation and histochemistry. J Neurol Sci. 1986;75:89–103. doi: 10.1016/0022-510x(86)90052-3. [DOI] [PubMed] [Google Scholar]
- 19.Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil. 1999;13:354–362. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- 20.Boissy P, Bourbonnais D, Kaegi C, Gravel D, Arsenault BA. Characterization of global synkineses during hand grip in hemiparetic patients. Arch Phys Med Rehabil. 1997;78:1117–1124. doi: 10.1016/s0003-9993(97)90138-6. [DOI] [PubMed] [Google Scholar]
- 21.Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, Dromerick AW. Deficits in grasp versus reach during acute hemiparesis. Exp Brain Res. 2005;166:126–136. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- 23.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 24.Beebe JA, Lang CE. Absence of a proximal to distal gradient of motor deficits in the upper extremity early after stroke. Clin Neurophysiol. 2008;119:2074–2085. doi: 10.1016/j.clinph.2008.04.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beebe JA, Lang CE. Active range of motion predicts upper extremity function 3 months after stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.108.536763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- 27.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 28.Schieber MH, Lang CE, Reilly KT, McNulty P, Sirigu A. Selective activation of human finger muscles after stroke or amputation. Adv Exp Med Biol. 2009;629:559–575. doi: 10.1007/978-0-387-77064-2_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 30.Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabil Neural Repair. 2007;21:279–291. doi: 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- 31.Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 32.Fredericks CM. Disorders of the peripheral nervous system: The peripheral neuropathies. In: Fredericks CM, Saladin LK, editors. Pathophysiology of the motor systems: Principles and clinical presentations. FA Davis; Philadelphia: 1996. pp. 346–372. [Google Scholar]
- 33.Hreib KK, Jones HR. Clinical neurologic evaluation. In: Jones HR, editor. Netter’s neurology. Icon Learning Systems; Teterboro, NJ: 2005. pp. 2–39. [Google Scholar]
- 34.Winward CE, Halligan PW, Wade DT. Current practice and clinical relevance of somatosensory assessment after stroke. Clin Rehabil. 1999;13:48–55. doi: 10.1177/026921559901300107. [DOI] [PubMed] [Google Scholar]
- 35.Kent BE. Sensory-motor testing: The upper limb of adult patients with hemiplegia. Phys Ther. 1965;45:550–561. doi: 10.1093/ptj/45.6.550. [DOI] [PubMed] [Google Scholar]
- 36.Lang CE, Dejong SL, Beebe JA. Recovery of thumb and finger extension and its relation to grasp performance after stroke. J Neurophysiol. 2009;102:451–459. doi: 10.1152/jn.91310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prager EM, Lang CE. Predictive ability of 2-day measurement of active range of motion on 3-mo upper extremity motor function in people with post-stroke hemiparesis. Am J Occup Ther. 2012;66:1–7. doi: 10.5014/ajot.2012.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bland MD, Beebe JA, Hardwick DD, Lang CE. Restricted active range of motion at the elbow, forearm, wrist, or fingers decreases hand function. J Hand Ther. 2008;21:268–274. doi: 10.1197/j.jht.2008.01.003. quiz 275. [DOI] [PubMed] [Google Scholar]
- 39.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 40.Baker K, Cano SJ, Playford ED. Outcome measurement in stroke: A scale selection strategy. Stroke. 2011;42:1787–1794. doi: 10.1161/STROKEAHA.110.608505. [DOI] [PubMed] [Google Scholar]
- 41.Ashford S, Slade M, Malaprade F, Turner-Stokes L. Evaluation of functional outcome measures for the hemiparetic upper limb: A systematic review. J Rehabil Med. 2008;40:787–795. doi: 10.2340/16501977-0276. [DOI] [PubMed] [Google Scholar]
- 42.Connell LA, Tyson SF. Clinical reality of measuring upper-limb ability in neurologic conditions: A systematic review. Arch Phys Med Rehabil. 2012;93:221–228. doi: 10.1016/j.apmr.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27:107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh YW, Wu CY, Lin KC, Chang YF, Chen CL, Liu JS. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke. 2009;40:1386–1391. doi: 10.1161/STROKEAHA.108.530584. [DOI] [PubMed] [Google Scholar]
- 46.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: Properties of the action research arm test. Arch Phys Med Rehabil. 2006;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Nijland R, van Wegen E, Verbunt J, van Wijk R, van Kordelaar J, Kwakkel G. A comparison of two validated tests for upper limb function after stroke: The wolf motor function test and the action research arm test. J Rehabil Med. 2010;42:694–696. doi: 10.2340/16501977-0560. [DOI] [PubMed] [Google Scholar]
- 49.van der Lee JH, Roorda LD, Beckerman H, Lankhorst GJ, Bouter LM. Improving the action research arm test: A unidimensional hierarchical scale. Clin Rehabil. 2002;16:646–653. doi: 10.1191/0269215502cr534oa. [DOI] [PubMed] [Google Scholar]
- 50.van der Lee JH, Beckerman H, Knol DL, de Vet HC, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410–1414. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 51.van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the action research arm test and the fugl-meyer assessment scale in chronic stroke patients. J Rehabil Med. 2001;33:110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 52.van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: A practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 53.Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the fugl-meyer test, action research arm test and box and block test: A multicentre study. Clin Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 54.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 55.Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the box and block test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75:751–755. [PubMed] [Google Scholar]
- 56.Lin KC, Chuang LL, Wu CY, Hsieh YW, Chang WY. Responsiveness and validity of three dexterous function measures in stroke rehabilitation. J Rehabil Res Dev. 2010;47:563–571. doi: 10.1682/jrrd.2009.09.0155. [DOI] [PubMed] [Google Scholar]
- 57.Barreca S, Gowland CK, Stratford P, Huijbregts M, Griffiths J, Torresin W, Dunkley M, Miller P, Masters L. Development of the chedoke arm and hand activity inventory: Theoretical constructs, item generation, and selection. Top Stroke Rehabil. 2004;11:31–42. doi: 10.1310/JU8P-UVK6-68VW-CF3W. [DOI] [PubMed] [Google Scholar]
- 58.Barreca S, Stratford P, Masters L, Lambert CL, Griffiths J, McBay C. Validation of three shortened versions of the chedoke arm and hand activity inventory. Physiother Can. 2006;58:1–9. [Google Scholar]
- 59.Barreca SR, Stratford PW, Lambert CL, Masters LM, Streiner DL. Test-retest reliability, validity, and sensitivity of the chedoke arm and hand activity inventory: A new measure of upper-limb function for survivors of stroke. Arch Phys Med Rehabil. 2005;86:1616–1622. doi: 10.1016/j.apmr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Barreca SR, Stratford PW, Masters LM, Lambert CL, Griffiths J. Comparing 2 versions of the chedoke arm and hand activity inventory with the action research arm test. Phys Ther. 2006;86:245–253. [PubMed] [Google Scholar]
- 61.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 62.Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine hole peg test of finger dexterity. The Occupational Therapy Journal of Research. 1985;5:24–38. [Google Scholar]
- 63.Croarkin E, Danoff J, Barnes C. Evidence-based rating of upper-extremity motor function tests used for people following a stroke. Phys Ther. 2004;84:62–74. [PubMed] [Google Scholar]
- 64.Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989;52:1267–1272. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: Measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry. 1987;50:714–719. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 67.Morris DM, Uswatte G, Crago JE, Cook EW, 3rd, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 68.Whitall J, Savin DN, Jr., Harris-Love M, Waller SM. Psychometric properties of a modified wolf motor function test for people with mild and moderate upper-extremity hemiparesis. Arch Phys Med Rehabil. 2006;87:656–660. doi: 10.1016/j.apmr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL. Minimal detectable change scores for the wolf motor function test. Neurorehabil Neural Repair. 2009;23:662–667. doi: 10.1177/1545968309335975. [DOI] [PubMed] [Google Scholar]
- 70.Wu CY, Fu T, Lin KC, Feng CT, Hsieh KP, Yu HW, Lin CH, Hsieh CJ, Ota H. Assessing the streamlined wolf motor function test as an outcome measure for stroke rehabilitation. Neurorehabil Neural Repair. 2011;25:194–199. doi: 10.1177/1545968310381249. [DOI] [PubMed] [Google Scholar]
- 71.Wolf SL, Thompson PA, Morris DM, Rose DK, Winstein CJ, Taub E, Giuliani C, Pearson SL. The excite trial: Attributes of the wolf motor function test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 72.Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Liu JS. Minimal detectable change and clinically important difference of the wolf motor function test in stroke patients. Neurorehabil Neural Repair. 2009;23:429–434. doi: 10.1177/1545968308331144. [DOI] [PubMed] [Google Scholar]
- 73.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The motor activity log-28: Assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 74.Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity motor activity log-14 for measuring real-world arm use. Stroke. 2005;36:2493–2496. doi: 10.1161/01.STR.0000185928.90848.2e. [DOI] [PubMed] [Google Scholar]
- 75.Dromerick AW, Lang CE, Birkenmeier R, Hahn MG, Sahrmann SA, Edwards DF. Relationships between upper-limb functional limitation and self-reported disability three months after stroke. J Rehabil Res Dev. 2006;43:401–408. doi: 10.1682/jrrd.2005.04.0075. [DOI] [PubMed] [Google Scholar]
- 76.Duncan PW, Lai SM, van Culin V, Huang L, Clausen D, Wallace D. Development of a comprehensive assessment toolbox for stroke. Clin Geriatr Med. 1999;15:885–915. [PubMed] [Google Scholar]
- 77.Duncan PW, Bode RK, Lai S Min, Perera S. Rasch analysis of a new stroke-specific outcome scale: The stroke impact scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/s0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 78.Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the stroke impact scale. Stroke. 2002;33:2593–2599. doi: 10.1161/01.str.0000034395.06874.3e. [DOI] [PubMed] [Google Scholar]
- 79.Duncan PW, Reker DM, Horner RD, Samsa GP, Hoenig H, LaClair BJ, Dudley TK. Performance of a mail-administered version of a stroke-specific outcome measure, the stroke impact scale. Clin Rehabil. 2002;16:493–505. doi: 10.1191/0269215502cr510oa. [DOI] [PubMed] [Google Scholar]
- 80.Duncan PW, Wallace D, Studenski S, Lai SM, Johnson D. Conceptualization of a new stroke-specific outcome measure: The stroke impact scale. Top Stroke Rehabil. 2001;8:19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT. [DOI] [PubMed] [Google Scholar]
- 81.Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- 82.Lin KC, Fu T, Wu CY, Hsieh YW, Chen CL, Lee PC. Psychometric comparisons of the stroke impact scale 3.0 and stroke-specific quality of life scale. Qual Life Res. 2010;19:435–443. doi: 10.1007/s11136-010-9597-5. [DOI] [PubMed] [Google Scholar]
- 83.Lin KC, Fu T, Wu CY, Wang YH, Liu JS, Hsieh CJ, Lin SF. Minimal detectable change and clinically important difference of the stroke impact scale in stroke patients. Neurorehabil Neural Repair. 2010;24:486–492. doi: 10.1177/1545968309356295. [DOI] [PubMed] [Google Scholar]
- 84.Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, Hsieh CL. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 85.Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. J Neurol Phys Ther. 2009;33:96–103. doi: 10.1097/NPT.0b013e3181a33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 87.Jobe JB. Cognitive processes in self-report. In: Turkann JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: Implications for research and practice. Lawrence Erlbaum Associates; Mahwah: 2000. pp. 25–28. [Google Scholar]
- 88.Bradburn NM, Rips LJ, Shevell SK. Answering autobiographical questions: The impact of memory and inference on surveys. Science. 1987;236:157–161. doi: 10.1126/science.3563494. [DOI] [PubMed] [Google Scholar]
- 89.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: Frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rand D, Eng JJ, Tang PF, Jeng JS, Hung C. How active are people with stroke?: Use of accelerometers to assess physical activity. Stroke. 2009;40:163–168. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- 92.Green LB. Assessment of habitual physical activity and paretic arm mobility among stroke survivors by accelerometry. Top Stroke Rehabil. 2007;14:9–21. doi: 10.1310/tsr1406-9. [DOI] [PubMed] [Google Scholar]
- 93.Chen KY, Bassett DR., Jr. The technology of accelerometry-based activity monitors: Current and future. Med Sci Sports Exerc. 2005;37:S490–500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- 94.Uswatte G, Miltner WH, Foo B, Varma M, Moran S, Taub E. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke. 2000;31:662–667. doi: 10.1161/01.str.31.3.662. [DOI] [PubMed] [Google Scholar]
- 95.Welk GJ, Schaben JA, Morrow JR., Jr. Reliability of accelerometry-based activity monitors: A generalizability study. Med Sci Sports Exerc. 2004;36:1637–1645. [PubMed] [Google Scholar]
- 96.Welk GJ. Principles of design and analyses for the calibration of accelerometry-based activity monitors. Med Sci Sports Exerc. 2005;37:S501–511. doi: 10.1249/01.mss.0000185660.38335.de. [DOI] [PubMed] [Google Scholar]
- 97.Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB. Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch Phys Med Rehabil. 2005;86:1498–1501. doi: 10.1016/j.apmr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 98.Uswatte G, Giuliani C, Winstein C, Zeringue A, Hobbs L, Wolf SL. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the extremity constraint-induced therapy evaluation trial. Arch Phys Med Rehabil. 2006;87:1340–1345. doi: 10.1016/j.apmr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Lang CE, Wagner JM, Edwards DF, Dromerick AW. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J Neurol Phys Ther. 2007;31:56–63. doi: 10.1097/NPT.0b013e31806748bd. [DOI] [PubMed] [Google Scholar]
- 100.Seitz RJ, Hildebold T, Simeria K. Spontaneous arm movement activity assessed by accelerometry is a marker for early recovery after stroke. J Neurol. 2011;258:457–463. doi: 10.1007/s00415-010-5778-y. [DOI] [PubMed] [Google Scholar]
- 101.Page SJ, Sisto SA, Levine P. Modified constraint-induced therapy in chronic stroke. Am J Phys Med Rehabil. 2002;81:870–875. doi: 10.1097/00002060-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 102.Reiterer V, Sauter C, Klosch G, Lalouschek W, Zeitlhofer J. Actigraphy--a useful tool for motor activity monitoring in stroke patients. Eur Neurol. 2008;60:285–291. doi: 10.1159/000157882. [DOI] [PubMed] [Google Scholar]
- 103.Rand D, Eng JJ. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabil Neural Repair. 2012;26:76–84. doi: 10.1177/1545968311408918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keil A, Elbert T, Edward T. Relation of accelerometer and emg recordings for the measurment of upper extremity movement. J Psychophysiology. 1999;13:77–82. [Google Scholar]
- 105.de Niet M, Bussmann JB, Ribbers GM, Stam HJ. The stroke upper-limb activity monitor: Its sensitivity to measure hemiplegic upper-limb activity during daily life. Arch Phys Med Rehabil. 2007;88:1121–1126. doi: 10.1016/j.apmr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 106.van der Pas SC, Verbunt JA, Breukelaar DE, van Woerden R, Seelen HA. Assessment of arm activity using triaxial accelerometry in patients with a stroke. Arch Phys Med Rehabil. 2011;92:1437–1442. doi: 10.1016/j.apmr.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 107.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 108.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part ii: Time course of recovery. The copenhagen stroke study. Arch Phys Med Rehabil. 1995;76:406–412. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- 109.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: A systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–1637. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 110.Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32:107–112. doi: 10.1161/01.str.32.1.107. [DOI] [PubMed] [Google Scholar]
- 111.Patel AT, Duncan PW, Lai SM, Studenski S. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. 2000;81:1357–1363. doi: 10.1053/apmr.2000.9397. [DOI] [PubMed] [Google Scholar]
- 112.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part i: Outcome. The copenhagen stroke study. Arch Phys Med Rehabil. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- 113.Olsen TS. Arm and leg paresis as outcome predictors in stroke rehabilitation. Stroke. 1990;21:247–251. doi: 10.1161/01.str.21.2.247. [DOI] [PubMed] [Google Scholar]
- 114.Teasell R, Foley N, Salter K, Boghal S, Jutai J, Speechley M. Evidence-based review of stroke rehabilitation. University of Western Ontario; London, Ontario: 2008. [Google Scholar]
- 115.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very early constraint-induced movement during stroke rehabilitation (vectors): A single-center rct. Neurology. 2009;73:195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shepherd RB. Exercise and training to optimize functional motor performance in stroke: Driving neural reorganization? Neural Plasticity. 2001;8:121–129. doi: 10.1155/NP.2001.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeJong G, Horn SD, Conroy B, Nichols D, Healton EB. Opening the black box of post-stroke rehabilitation: Stroke rehabilitation patients, processes, and outcomes. Arch Phys Med Rehabil. 2005;86:S1–S7. doi: 10.1016/j.apmr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Collin C, Wade D. Assessing motor impairment after stroke: A pilot reliability study. J Neurol Neurosurg Psychiatry. 1990;53:576–579. doi: 10.1136/jnnp.53.7.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19:382–389. doi: 10.1159/000115178. [DOI] [PubMed] [Google Scholar]
- 121.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 122.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 123.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 124.Veerbeek JM, Van Wegen EE, Harmeling-Van der Wel BC, Kwakkel G. Is accurate prediction of gait in nonambulatory stroke patients possible within 72 hours poststroke? The epos study. Neurorehabil Neural Repair. 2011;25:268–274. doi: 10.1177/1545968310384271. [DOI] [PubMed] [Google Scholar]
- 125.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 126.Thrane G, Emaus N, Askim T, Anke A. Arm use in patients with subacute stroke monitored by accelerometry: Association with motor impairment and influence on self-dependence. J Rehabil Med. 2011;43:299–304. doi: 10.2340/16501977-0676. [DOI] [PubMed] [Google Scholar]
- 127.Gebruers N, Vanroy C, Truijen S, Engelborghs S, De Deyn PP. Monitoring of physical activity after stroke: A systematic review of accelerometry-based measures. Arch Phys Med Rehabil. 91:288–297. doi: 10.1016/j.apmr.2009.10.025. [DOI] [PubMed] [Google Scholar]