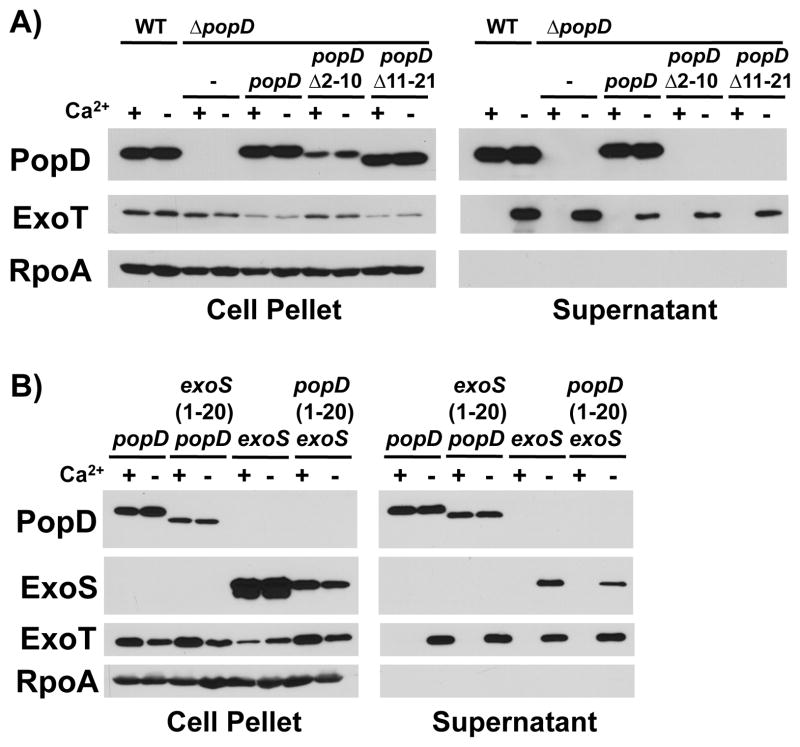
Fig. 1. The N-terminal secretion signal does not direct export of translocators before effectors.
A) Export of PopD lacking amino acid residues 2–10 or 11–21 was assayed in the presence and absence of calcium by complementing a popD deletion mutant (ΔpopD: PAO1F ΔfleQ ΔpopD) with plasmids expressing either wild-type PopD, or the corresponding mutant protein and compared to export of PopD expressed from its native chromosomal location (WT: PAO1F ΔfleQ). Cell pellet and supernatant fractions were probed with antibodies directed against PopD, the effector ExoT, as well as the RNA polymerase alpha subunit (RpoA, fractionation control).
B) PopD, ExoS, as well as proteins in which the secretion signals of the two proteins had been exchanged [ExoS(1-20)-PopD(21-295) and PopD(1-20)-ExoS(21-453)] were expressed in a strain lacking the chromosomal copies of exoS, popB and popD (PAO1F ΔfleQ ΔexoS ΔpopBD). Export was assayed in the presence and absence of calcium. Cell pellet and supernatant fractions were probed with antibodies directed against PopD, RpoA, as well as the effectors ExoS and ExoT.