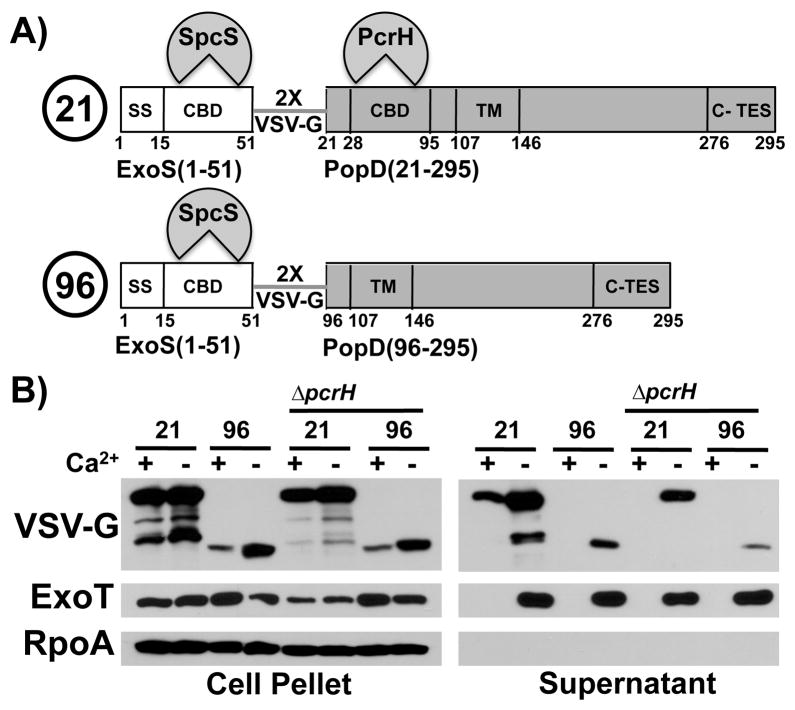
Fig. 5. PcrH and the chaperone binding domain are required for export of PopD before effectors.
A) Schematic representation of the two fusion proteins. ExoS(1-51, white) is fused to PopD (21-295, grey, “21”) or PopD (96-295, grey, “96”). The two fusion partners are joined by a linker consisting of two copies of the VSV-G protein epitope tag. The secretion signal of ExoS (SS) as well as the chaperone binding domains of ExoS and PopD (CBD) are indicated. The chaperone that binds to each of the cognate chaperone binding domains is also indicated (SpcS, the export chaperone of ExoS and ExoT as well as PcrH, the export chaperone of PopB and PopD). The location of the C-TES (TES) is noted as well. Amino acid numbers corresponding do the domain boundaries in the native protein are indicated below.
B) Two fusion proteins, ExoS(1-51)-VSV-G-PopD(21-295) (labeled 21), and ExoS(1-51)-VSV-G-popD(96-295) (labeled 96) were expressed in trans in PAO1F ΔfleQ ΔexoS ΔpopBD and PAO1F ΔfleQ ΔexoS ΔpcrHpopBD(ΔpcrH). Export was assayed in the presence and absence of calcium. Cell pellet and supernatant fractions were probed with antibodies directed against VSV-G(ExoS-PopD hybrid), RpoA, as well as the effector ExoT.