Abstract
The major aims of this study were to estimate the infection rate and recognize the risk factor for ventriculoperitoneal (VP) shunt infections in children. To analyze shunt infection rate and identify risk factors, a retrospective cohort analysis of 333 consecutive VP shunt series was performed at Seoul National University Children's Hospital in Korea between January 2005 and February 2011. Overall, 35 shunts (10.5%) were infected, which represented an infection rate of 0.075 infection cases per shunt per year. VP shunt infection occurred at a median of 1 month (range, 6 days to 8 months) after insertion. An independent risk factor for shunt infection was undergoing an operation before the first year of life (relative risk 2.31; 95% confidence interval, 1.19-4.48). The most common causative microorganism was coagulase-negative staphylococci in 16 (45.7%) followed by Staphylococcus aureus in 8 (22.9%). Methicillin resistance rate was 83.3% among coagulase-negative staphylococci and S. aureus. In this study, cerebrospinal fluid shunt infection rate was 10.5%. Infection was frequently caused by methicillin-resistant coagulase-negative staphylococci and S. aureus within two months after shunt surgery. Vancomycin may be considered as the preoperative prophylaxis for shunt surgery in a situation where methicillin resistance rate is very high.
Keywords: Ventriculoperitoneal Shunt, Infection, Prophylaxis, Child
INTRODUCTION
Hydrocephalus is caused by the inability of cerebrospinal fluid (CSF) to drain into the bloodstream. Ventriculoperitoneal (VP) shunt is used in the setting of hydrocephalus to divert CSF to the peritoneum. Although VP shunts have resulted in a dramatic improvement in patient survival and neurological function, CSF shunts are associated with several complications (1). One of the most common and critical complications is infection, which occurs in 3% to 20% of cases (2-4). The results of risk factor analyses have varied, however, the following risk factors for infection were identified: shunt insertion at a younger age, patient prematurity, and the presence of a postoperative CSF leak (3, 5).
This study estimated the infection rate of the consecutive shunt series in children over 6 yr at a single center and investigated the risk factors, bacterial pathogens and their antimicrobial susceptibility patterns, the treatment modalities, and clinical outcome, to guide better clinical practice in the management of VP shunt in children.
MATERIALS AND METHODS
Patients and methods
All of the pediatric patients who underwent a VP shunt insertion for treatment of hydrocephalus at Seoul National University Children's Hospital over 6 yr between January 2006 and February 2011 were registered on the operation directory of division of pediatric neurosurgery. The following data were collected from the retrospective review of patient records: demographic findings; age at operation; follow-up period; shunt characteristics, such as type and number; external ventricular drain (EVD) use; antibiotics used for perioperative prophylaxis; etiology of hydrocephalus; other predisposing medical conditions; symptoms exhibited at the time of infection; blood and CSF culture results and other laboratory results; treatment modality, including type and duration of antibiotics; and final outcomes.
Definitions and criteria for infection
The criteria and classification for CSF shunt infection were as follows. Documented infection was defined as the identification of a bacterial pathogen from the reservoir CSF or reservoir CSF pleocytosis (more than 50 leukocytes per cubic millimeter) in association with positive blood culture when a patient presented any of the following: fever, neurologic symptoms, abdominal symptoms, or shunt malfunction. Probable infection was defined as reservoir CSF pleocytosis in the cases who presented with fever and neurologic symptoms although CSF culture did not grow a pathogen (4, 6).
Treatment of shunt infection
When shunt infection was suspected, we used a management protocol which was previously suggested with minor modification (7). Briefly, vancomycin was empirically initiated right after CSF reservoir tapping was performed. The antibiotics were changed based on the CSF and/or blood culture and antibiotic susceptibility results, if necessary. Based on a consensus to remove infected CSF shunts, the mainstay of treatment included shunt removal for all of the patients. EVD and/or externalization of the shunt were considered while the patients were treated with antibiotics. CSF shunt was re-inserted after a proper duration of treatment, usually 10 days after negative conversion of CSF culture results.
Risk factor analysis
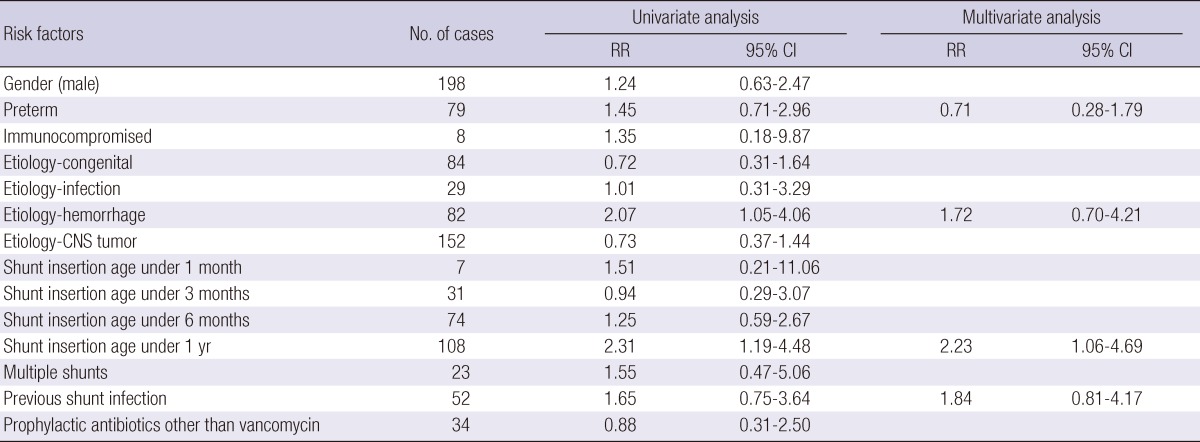
Risk factors for shunt infection were evaluated including gender, age at the time of shunt insertion, multiple shunts, a history of shunt infection, preterm birth, immune status, and the etiology of hydrocephalus. The age at shunt insertion was analyzed by dividing into the following 4 groups: less than 1 month, less than 3 months, less than 6 months, and less than 1 yr. The Cox proportional hazard ratio and relative risk were estimated. In the univariate analysis, each risk factor was analyzed separately to measure the relative risk. Multivariate analysis included factors that indicated statistical significance in the univariate analysis, previous shunt infection, and the factors that could influence each other, such as intraventricular hemorrhage and preterm birth. The statistical analysis was performed using SPSS, version 19.0 (IBM®).
Ethics statement
This study was approved by the institutional review board of Seoul National University Hospital (Approval Number: H-1106-084-366). Informed consent was exempted by the board.
RESULTS
Patient population
From January 2005 to February 2011, a total of 333 CSF shunts were inserted into 224 pediatric patients. The etiology of hydrocephalus was as follows: central nervous system (CNS) tumor, 101 patients (45.1%); congenital, 61 patients (27.2%); intraventricular hemorrhage (IVH) or subdural hemorrhage (SDH), 50 patients (22.3%); infection (e.g. meningitis or encephalitis), 19 patients (8.5%); trauma, 3 patients (1.3%); and unknown, 5 shunt insertions (2.2%). Overall, 198 shunts (59.5%) were placed into male patients. The median age of the patients at the time of shunt insertion was 11 months (range, 1 day to 193 months). A total of 79 VP shunts (23.7%) were inserted into premature neonates. Of the 333 inserted shunts, 202 shunts (60.7%) were first-inserted shunts and 131 (39.3%) were re-inserted shunts. The median duration of follow-up was 8 months (range, 1 day to 72 months).
Of the 333 shunt insertions, perioperative antibiotics were used with the exception of one case. The major prophylactic antibiotics were first generation cephalosporin in 271 cases (81.4%) and vancomycin in 36 cases (10.8%).
Incidence of VP shunt infection
Of the 333 inserted shunts, 35 shunts (10.5%) became infected, which represented an infection rate of 0.075 infection cases per shunt per year. By definition, 31 cases (88.6%) out of the 35 infection cases were documented infection, and 4 cases (11.4%) were probable infection. The prevalence of shunt infection according to primary etiology was as follows: CNS tumor, 13 (8.6%) of 152 cases; congenital, 8 (9.1%) of 87 cases; IVH or SDH, 14 (17.1%) of 82 cases; meningitis or encephalitis, 3 (10.3%) of 29 cases; and none in 3 cases with trauma.
Insertion-to-infection duration
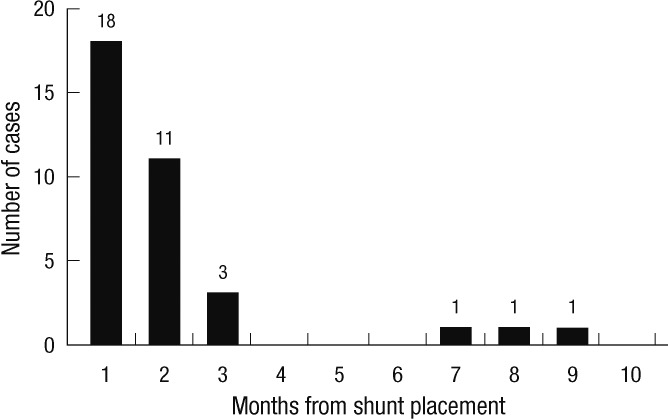
VP shunt infection occurred at a median of 1 month (range, 6 days to 8 months) after insertion. Of the 35 shunt infections, 18 infections (51.4%) occurred within 1 month of shunt insertion, and 32 infections (91.4%) occurred within 3 months of shunt insertion (Fig. 1). There was no significant difference in the insertion-to-infection duration between methicillin-susceptible and non-susceptible strain (median of 38 vs 38.5 days, P = 0.16).
Fig. 1.
Time interval from shunt placement to infection. Of the 35 shunt infections, 18 infections (51.4%) occurred within 1 month of shunt insertion, and 32 infections (91.4%) occurred within 3 months of shunt insertion (range 6 days to 8 months).
Microbiology
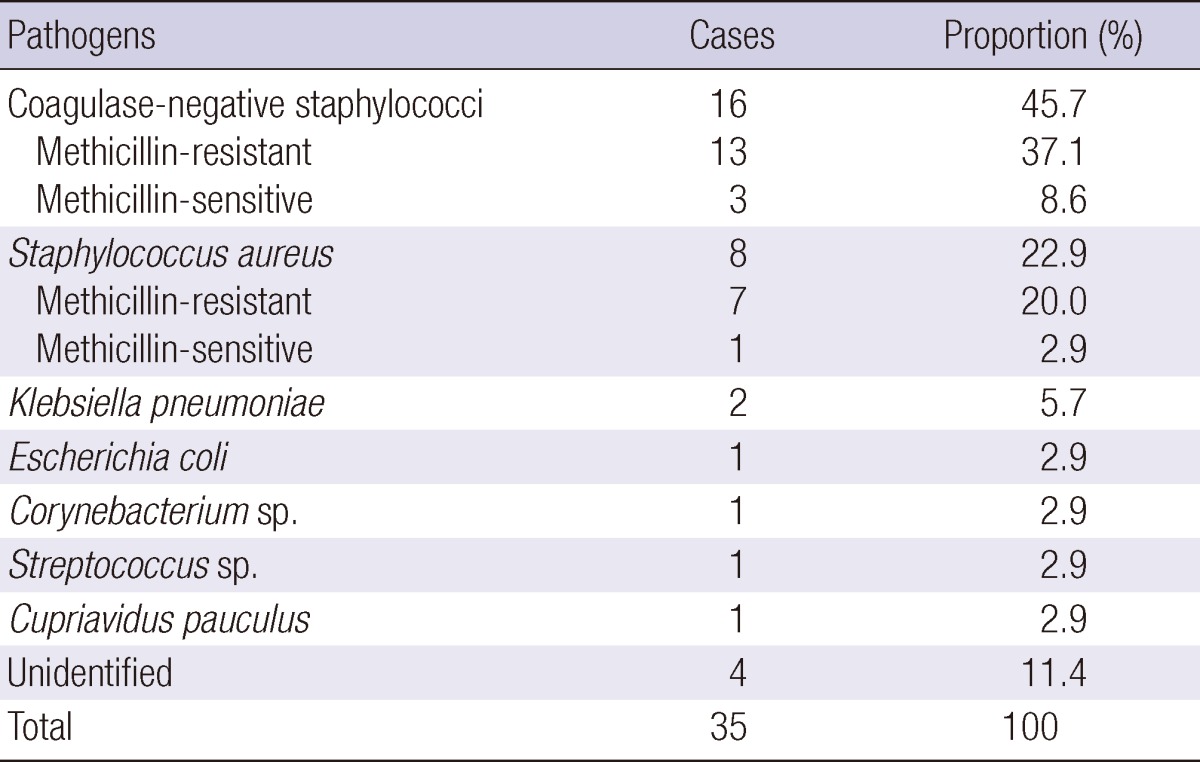
Excluding 4 cases (11.4%) of probable infections, a microbial pathogen was identified in 31 out of 35 shunt infections (88.6%). The most common pathogen was coagulase-negative staphylococci, in 16 cases (45.7%) followed by Staphylococcus aureus, in 8 cases (22.9%). These two pathogens accounted for approximately 70% of the shunt infections. Other pathogens that were identified in the culture results were as follows: 2 cases of Klebsiella pneumoniae (5.7%); and each case of Bacillus sp. (2.9%), Corynebacterium sp. (2.9%), Streptococcus sp. (2.9%), Cupriavidus pauculus (2.9%), and Escherichia coli (2.9%) (Table 1). Thirteen of 16 coagulase-negative staphylococci (81.2%) and 7 of 8 S. aureus cases (87.5%) were resistant to methicillin. Etiologic organisms of the preceding infectious conditions such as meningitis or encephalitis were different from those of shunt infection. Despite the use of vancomycin as the prophylactic antibiotics in 36 cases, there were 4 infection cases caused by methicillin-resistant S. aureus (n = 2) and methicillin-resistant S. epidermidis (n = 2). There was no statistical difference in the duration of prophylactic antibiotic use between methicillin-susceptible and non-susceptible strain (median of 5 days vs 8.5 days, P = 0.48).
Table 1.
Pathogens identified in VP shunt infection
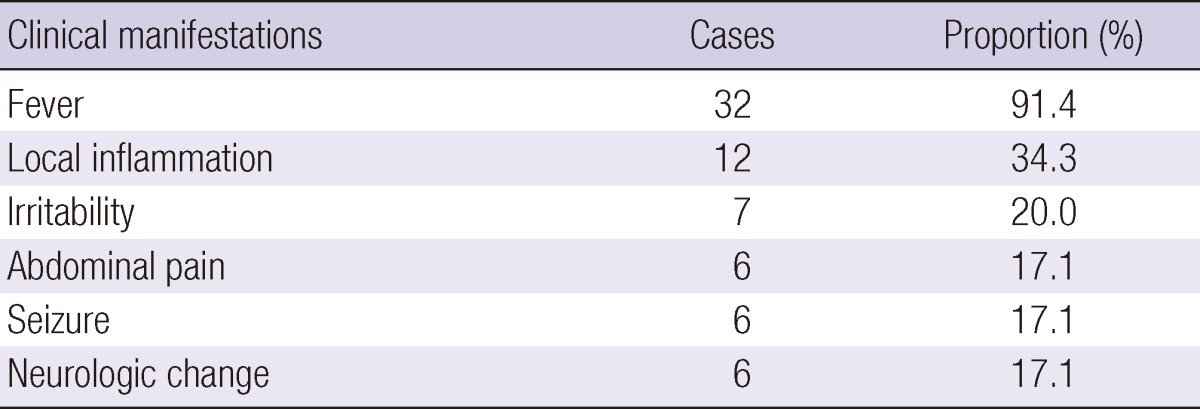
Clinical manifestations
The most common symptoms of shunt infection were the following: fever, 32 patients (91.4%); local inflammation (e.g., local tenderness, swelling, heat, induration and shunt malfunction), 12 patients (34.3%); irritability, 7 patients (20.0%); abdominal pain, 6 patients (17.1%); seizure, 6 patients (17.1%); and neurologic abnormality, 6 patients (17.1%) (Table 2).
Table 2.
Clinical symptoms in 35 patients with VP shunt infection
Risk factors for shunt infection
In the univariate analysis, a shunt that was performed on a patient under the age of 1 yr (relative risk [RR], 2.31; 95% confidence interval [CI], 1.19-4.48) and the presence of hydrocephalus due to hemorrhage (RR, 2.07; 95% CI, 1.05-4.06) demonstrated statistical significance. Multivariate analysis included factors that suggested statistical significance in the univariate analysis and the factors that could influence each other, such as intraventricular hemorrhage and preterm birth. Multivariate analysis demonstrated that shunt insertion on a patient under the age of 1 yr was an independent risk factor (RR, 2.23; 95% CI, 1.06-4.69) (Table 3).
Table 3.
Analysis of the risk factors for VP shunt infection
The risk factors for VP shunt infection were analyzed with Cox proportional hazards analysis, in univariate and multivariate analyses using SPSS, version 19.0 (IBM®). RR, relative risk; CI, confidence interval.
Infection of the re-inserted shunts
Of the 333 shunt insertions, 131 cases (39.3%) involved shunts that had been re-inserted after a previous shunt removal. The infection rate of the re-inserted shunts was 13.0% (17 of 131 shunts) compared to that of the first-inserted shunts, 8.9% (18 of 202 shunts). Two main reasons for re-insertion were shunt malfunction in 99 (75.6%) and infection of the previous shunts in 29 (22.1%). Shunt infection rates by the cause of shunt re-insertion were as follows: 17.2% (5 of 29) in prior infection group and 12.1% (12 of 99) in malfunction group. Although there was a tendency of high infection rate (17.2%) of the re-inserted shunts due to prior infection compared to that of the first-inserted shunts (8.9%), there was no statistical significance in the comparison. Reinfections occurred in 4 re-inserted shunts due to prior shunt infection. Among those, same pathogen was found in only one case.
Treatment outcomes
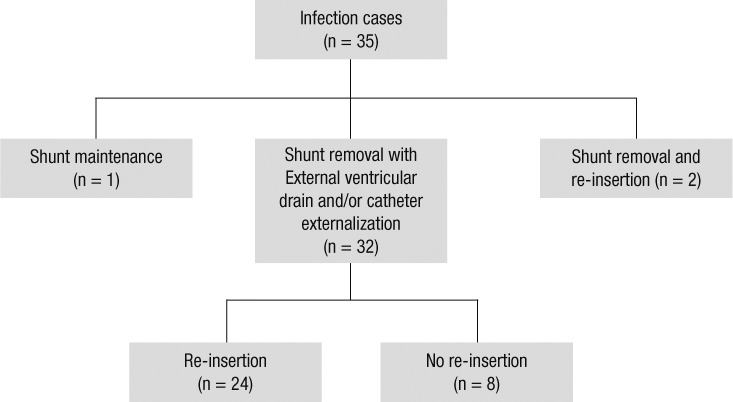
Of the 35 infection cases, 34 cases underwent shunt removal, excluding 1 case in which treatment was interrupted by a parental demand. Of the 34 cases with shunt removal, 26 cases (76.5%) underwent shunt re-insertion. The surgical procedures performed with shunt removal were as follows: EVD insertion in 22 cases (68.8%); externalization of the shunt in 3 cases (9.4%); and externalization of the shunt and subsequent EVD insertion in 7 cases (21.9%) (Fig. 2).
Fig. 2.
Surgical management of shunt infection. External ventricular drain in 22 cases (68.8%); externalization of the shunt in 3 cases (9.4%); and both of these procedures in 7 cases (21.9%).
Initial antibiotics at the time of shunt infection included either vancomycin alone or vancomycin plus other antibiotic agents in all cases. According to the antibiotic susceptibility, initial antibiotics were appropriate in 25 patients (80.6%) of 31 cases with identified pathogens. The median duration of antibiotic use was 26 days (range, 13 to 57 days), and the duration from a positive culture to negative culture conversion was 6 days (range, 2 to 35 days). Overall, a 5 yr old female patient with an underlying brain stem glioma died of shunt infection caused by Klebsiella pneumoniae which was accompanied with bacteremia.
DISCUSSION
Although CSF shunts contributed to the significant improvement of the management outcome of hydrocephalus, the shunt has several complications, including infection, which is a major threat to pediatric patients (8). This study demonstrated that infection occurred in 35 cases (10.5%) of the 333 consecutive shunt cases who underwent VP shunt insertion at a single center over 6 yr from January 2005 to February 2011. The rate of VP shunt infection in neurosurgical units is reported from 3% to 20% (2-4, 9). Many studies have suggested that coagulase-negative staphylococci are the major pathogens in shunt infections, followed by S. aureus (4, 10-12). However, gram-negative bacteria are also responsible for 7%-24% of all VPS infections (7, 13). This study is consistent with previous reports of shunt infections that were caused by opportunistic pathogens which had colonized the skin of the patient (12).
As shown in the previous reports, this study also demonstrated that coagulase-negative staphylococci (45.7%) and S. aureus (22.9%) were most commonly isolated pathogens and gram-negative bacteria were relatively uncommon. However, it is important to note that methicillin resistance rate has significantly increased among these two major pathogens, coagulase-negative staphylococci and S. aureus, over the last ten years. Methicillin resistance rate (20 of 24, 83.3%) among staphylococci was higher than that (7 of 19, 36.8%) of prior study conducted at the same institute between 1988 and 1995 (7). It is important to emphasize strict adherence to sterile technique during neurosurgical procedures to prevent shunt infection. It has also been shown that perioperative antibacterial prophylaxis has contributed to the prevention of infection (9). Currently, our standard practice is the use of first generation cephalosporins as the perioperative antibacterial prophylaxis. We were not able to demonstrate definite benefit of vancomycin prophylaxis in this study (infection rate of 10.3% in the 271 cases who were given with first generation cephalosporin versus 11.1% in the 36 cases given with vancomycin, P = 0.77). However, statistical power was low probably due to small number of cases in vancomycin use. However, given the high prevalence of methicillin resistance rate among staphylococci and the finding that interval from shunt insertion to infection is usually within 2 months, role of first generation cephalosporins as the prophylactic agent is questionable, especially when infection occurs in association with colonized skin flora shortly after shunt surgery. Although it is controversial to use vancomycin as a prophylactic agent in shunt surgery (14), there is a randomized prospective control study that showed that vancomycin was more effective than cefazolin in decreasing shunt infections in adult patients (4% vs 14% in infection rate) (15).
In this study, a major risk factor for shunt infection was receiving a shunt insertion at under one year of age. This association may be due to the poorly developed humoral and cellular immune systems in infants under 1 yr of age, the immaturity of the skin barrier in early infants, and the features of residential bacterial flora in this age group. In addition, improvements in neonatal intensive care have reduced the mortality rates of infants with an extremely low birth weight who are immunologically vulnerable and who have a significantly higher risk of developing IVH, which increases the necessity of a VP shunt at a younger age (16). Risk factors for shunt infection include preterm infants, younger age of the patient at shunt placement, hydrocephalus as a consequence of IVH or meningitis (2, 3, 5). Other potential risk factors include postoperative CSF leaks, the frequency of manual contacts between the neurosurgeon and the shunt system, the duration of the surgical procedure, the experience of the surgeon, or the use of a single glove versus the intraoperative double-gloving strategy (5, 17).
It has been known that re-inserted shunts due to previous infection may have a greater chance of becoming infected (18, 19). However, there is no study to compare infection rate between new shunts and re-inserted shunts. As this study included all consecutive shunt series, we were able to compare infection rate according to the presence of re-inserted shunts and etiology for shunt insertion. There was a tendency of high infection rate (17.2%) of the re-inserted shunts due to prior infection compared to that of the first-inserted shunts (8.9%). Unfortunately, this tendency did not reach statistical significance due to insufficient statistical power.
This study has several limitations. First, this study was not a prospective cohort analysis and has limitations in evaluating the neurologic differences before and after treatment. Second, we were not able to demonstrate difference in the prevention of shunt infection between first generation cephalosporin and vancomycin as the perioperative prophylactic regimen. This was probably owing to small number of cases in vancomycin-treated group and cases with heterogenous clinical parameters. Further studies are needed to evaluate efficacy of vancomycin prophylaxis in prevention of shunt infection and possible emergence of vancomycin resistance in the setting of high prevalence of methicillin-resistant staphylococci.
Prevention, early detection and immediate treatment reduce the morbidity and mortality in patients who suffer from this severe complication of shunt placement (13). Perioperative antibacterial prophylaxis and improvements in neurosurgical procedures are crucial to lower the incidence of VP shunt infection. We found several important observations: a significant increase in methicillin resistance rate among the major causative agents, more infections with methicillin-resistant strains after shunt insertion due to hydrocephalus following hemorrhage, identification of groups in high-risk, and comparison of infection rate between the new shunts and re-inserted shunts. Increased risk of shunt infection for receiving a shunt insertion at under one year of age implies that careful attention should be taken for this recognized high risk group and if possible efforts should be made to avoid shunt insertion for this specific group.
Based on the findings of high prevalence rate of methicillinresistant staphylococci, we suggest that vancomycin may be the appropriate agent as the perioperative prophylaxis, at least for those who will have the shunts re-inserted due to prior infection and who have shunt insertions after hydrocephalus due to hemorrhage. Also pursuit for novel strategies such as the use of antimicrobially-active shunt materials is needed (20-22).
References
- 1.Hirsch JF. Surgery of hydrocephalus: past, present and future. Acta Neurochir (Wien) 1992;116:155–160. doi: 10.1007/BF01540869. [DOI] [PubMed] [Google Scholar]
- 2.Davis SE, Levy ML, McComb JG, Masri-Lavine L. Does age or other factors influence the incidence of ventriculoperitoneal shunt infections? Pediatr Neurosurg. 1999;30:253–257. doi: 10.1159/000028806. [DOI] [PubMed] [Google Scholar]
- 3.Dallacasa P, Dappozzo A, Galassi E, Sandri F, Cocchi G, Masi M. Cerebrospinal fluid shunt infections in infants. Childs Nerv Syst. 1995;11:643–648. doi: 10.1007/BF00300722. [DOI] [PubMed] [Google Scholar]
- 4.Odio C, McCracken GH, Jr, Nelson JD. CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138:1103–1108. doi: 10.1001/archpedi.1984.02140500009004. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 6.McClinton D, Carraccio C, Englander R. Predictors of ventriculoperitoneal shunt pathology. Pediatr Infect Dis J. 2001;20:593–597. doi: 10.1097/00006454-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wang KC, Lee HJ, Sung JN, Cho BK. Cerebrospinal fluid shunt infection in children: efficiency of management protocol, rate of persistent shunt colonization, and significance of 'off-antibiotics' trial. Childs Nerv Syst. 1999;15:38–43. doi: 10.1007/s003810050324. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Wang KC, Cho BK. Functioning periods and complications of 246 cerebrospinal fluid shunting procedures in 208 children. J Korean Med Sci. 1995;10:275–280. doi: 10.3346/jkms.1995.10.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotim K, Miklic P, Paladino J, Melada A, Marcikic M, Scap M. Reducing the incidence of infection in pediatric cerebrospinal fluid shunt operations. Childs Nerv Syst. 1997;13:584–587. doi: 10.1007/s003810050144. [DOI] [PubMed] [Google Scholar]
- 10.Enger PØ, Svendsen F, Sommerfelt K, Wester K. Shunt revisions in children: can they be avoided? Experiences from a population-based study. Pediatr Neurosurg. 2005;41:300–304. doi: 10.1159/000088732. [DOI] [PubMed] [Google Scholar]
- 11.Kontny U, Höfling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ. CSF shunt infections in children. Infection. 1993;21:89–92. doi: 10.1007/BF01710738. [DOI] [PubMed] [Google Scholar]
- 12.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36:858–862. doi: 10.1086/368191. [DOI] [PubMed] [Google Scholar]
- 13.Stamos JK, Kaufman BA, Yogev R. Ventriculoperitoneal shunt infections with gram-negative bacteria. Neurosurgery. 1993;33:858–862. doi: 10.1227/00006123-199311000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Shah SS, Sinkowitz-Cochran RL, Keyserling HL, Jarvis WR. Vancomycin use in pediatric neurosurgery patients. Am J Infect Control. 1999;27:482–487. doi: 10.1016/s0196-6553(99)70025-8. [DOI] [PubMed] [Google Scholar]
- 15.Tacconelli E, Cataldo MA, Albanese A, Tumbarello M, Arduini E, Spanu T, Fadda G, Anile C, Maira G, Federico G, et al. Vancomycin versus cefazolin prophylaxis for cerebrospinal shunt placement in a hospital with a high prevalence of meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2008;69:337–344. doi: 10.1016/j.jhin.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Zafar N, Wallace CM, Kieffer P, Schroeder P, Schootman M, Hamvas A. Improving survival of vulnerable infants increases neonatal intensive care unit nosocomial infection rate. Arch Pediatr Adolesc Med. 2001;155:1098–1104. doi: 10.1001/archpedi.155.10.1098. [DOI] [PubMed] [Google Scholar]
- 17.Tulipan N, Cleves MA. Effect of an intraoperative double-gloving strategy on the incidence of cerebrospinal fluid shunt infection. J Neurosurg. 2006;104:5–8. doi: 10.3171/ped.2006.104.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, Muszynski C, Walker ML. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105:177–181. doi: 10.3171/ped.2006.105.3.177. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni AV, Rabin D, Lamberti-Pasculli M, Drake JM. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg. 2001;35:66–71. doi: 10.1159/000050393. [DOI] [PubMed] [Google Scholar]
- 20.Sciubba DM, Stuart RM, McGirt MJ, Woodworth GF, Samdani A, Carson B, Jallo GI. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg. 2005;103:131–136. doi: 10.3171/ped.2005.103.2.0131. [DOI] [PubMed] [Google Scholar]
- 21.Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst. 2007;23:773–777. doi: 10.1007/s00381-007-0296-7. [DOI] [PubMed] [Google Scholar]
- 22.Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007;7:38. doi: 10.1186/1471-2334-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]