Abstract
In this paper we report the results of an experiment to investigate the emergence of ocular vestibular evoked myogenic potentials (OVEMPs) during the linear vestibular ocular reflex (LVOR) evoked by whole-body vibration (WBV). OVEMP and electrooculogram (EOG) montages were employed to record periocular potentials (POPs) from six subjects during WBV in the nasooccipital (NO) axis over a range of frequencies from 0.5 to 64 Hz with approximately constant peak head acceleration of 1.0 ms−2 (i.e., 0.1 g). Measurements were made in two context conditions: a fixation context to examine the effect of gaze eccentricity (0 vs. 20°), and a visual context, where a target was either head-fixed or earth-fixed. The principal results are that from 0.5 to 2 Hz POP magnitude in the earth-fixed condition is related to head displacement, so with constant acceleration at all frequencies it reduces with increasing frequency, but at frequencies greater than 2 Hz both POP magnitude and POP gain, defined as the ratio of POP magnitude at 20 and 0°, increase with increasing frequency. By exhibiting this high-pass characteristic, a property shared with the LVOR, the results are consistent with the hypothesis that the OVEMP, as commonly employed in the clinical setting, is a high-frequency manifestation of the LVOR. However, we also observed low-frequency acceleration following POPs in head-fixed conditions, consistent with a low-frequency OVEMP, and found evidence of a high-frequency visual context effect, which is also consistent with the OVEMP being a manifestation of the LVOR.
Keywords: otoliths, ocular VEMP, vibration
the translational or linear vestibular-ocular reflexes (LVORs) play an essential role in assisting stabilization of binocular foveal images in a situation where the head is undergoing acceleration attributable to locomotion, orientation, or in response to unexpected perturbation. The LVOR is typically assessed by measuring eye movements during head acceleration, which may be achieved by various methods, including by camera, by eye coils, or by the electrooculogram (EOG), which records the periocular electrical activity associated with displacement of the retinal-corneal dipole. For vertical movements, the EOG is measured by means of a pair of surface electrodes located superior and inferior to the eye. The use of EOG methods is made difficult, however, by the fact that there are multiple sources of periocular potentials (POPs) associated with the LVOR. Among the additional POP sources is the electrical activity of the extraocular eye muscles stimulated by inputs from the vestibular system. These are referred to as ocular vestibular evoked myogenic potentials (OVEMPs) and most commonly measured from a differential pair of surface electrodes inferior to the eye. However, as OVEMPs may be excitatory (negative) or inhibitory (positive) they may not necessarily co-occur with an eye movement. The relationship between the EOG, OVEMPs, and the LVOR are not well understood, not least because OVEMPs are typically recorded using stimulus frequencies much higher than those encountered in experiments on the LVOR or which make use of EOG. For this reason we wished to investigate OVEMP responses at more typical LVOR frequencies.
Since the OVEMP and its physiological interpretation were first publicly announced (18, 22, 23, 27, 28) there has amassed a sizeable literature devoted to its exploitation (see 2, 19 for reviews). It is generally agreed that the OVEMP when activated by acoustic stimulation is a manifestation of the otolith-ocular pathways, but different modes of acoustic stimulation produce different patterns of end-organ activation (22). There is evidence that high-frequency air-conducted (AC) sound stimulation (best frequency 500 to 1,000 Hz) is selective for the saccule, whereas low-frequency vibration of the head (best frequency 80 to 100 Hz) appears to be more selective for the utricle, especially if the direction of vibration is aligned within the plane of morphological polarization of utricular hair-cells (24–26). Recent work by Zhang et al. (32, 33) has provided evidence that both sound and vibration may produce distinct resonances at ∼100 and 500 Hz, suggestive that the two resonance peaks are not specific to the two modes of stimulation but to the different dynamic responses of the two end organs.
One property of the OVEMP that is not controversial is that it is strongly modulated by gaze, so that if measured in an inferior location a large response can be obtained with upwards gaze, especially for AC stimulation (18, 24). For low-frequency vibration, upwards gaze also produces a large inferior response, but it is not abolished with downwards gaze, only altered in morphology, indicative of an alternative source (24). Given the role of the inferior oblique as an elevator and its particular anatomical location it is generally thought that the inferior OVEMP is produced primarily by this muscle with upwards gaze (8, 18, 22, 30, 31). Other muscles, including inferior, superior, and lateral recti have been suggested as sources for OVEMPs measured in other periocular locations and with differing gaze positions (25, 30).
Different assumptions about the mechanisms of end-organ activation lead to differing interpretations of the properties of OVEMPs. This is also attributable to the absence of a clear framework with which to relate acoustically activated otolith-ocular projections to their normal vestibular function as mediators of the LVOR. The end-organ receptors of the LVOR pathways must distinguish linear head accelerations in three possible head-fixed axes, a nasooccipital (NO) or x-axis, an interaural (IA) or y-axis, and a caudal-rostral (CA) or z-axis, against a background terrestrial gravitational acceleration giving a resultant gravito-inertial vector (GIV). The effectors of the LVOR pathways are required to produce two distinct corrective horizontal or vertical eye movements, vergence and version, and to produce torsional eye movements to counter tilt or equivalent tilt. In addition, the gain of the LVOR transformation must also be sensitive to changes in both viewing distance and gaze eccentricity, thus requiring a control system, which has access to eye position and state of vergence. On top of these already complex requirements the LVOR system must also integrate information from other sensory systems, not least the visual system during visual-vestibular interaction (VVI).
The LVOR systems and their role in the VVI have been the subject of experimental and theoretical examination in the last decade (4, 12–16). In these experiments, animal or human subjects are made to undergo translational accelerations and the eye position is recorded in various stimulus, visual, and gaze contexts. It has been established that the LVOR systems are essentially high pass in their sensitivity to acceleration at frequencies greater than 1 Hz, i.e., the gain increases with increasing frequency above 1 Hz. In contrast the visual pursuit system is low pass, i.e., the gain decreases with increasing frequency and dominates at stimulus frequencies less than ∼1 Hz (15). Thus at frequencies <1 Hz the visual system can suppress the LVOR for a head-fixed target, but at frequencies >1 Hz the visual system has less influence. In the range 1–10 Hz, the gain of the LVOR increases with increasing frequency, approaching, but not reaching, ideal gain at the high-frequency end of this range and is strongly modulated by viewing distance and gaze eccentricity, appropriate to achieve the correct vergence and version movements (12).
Viewing the OVEMP, LVOR, and VVI literature together, the essential difficulty in relating them directly is that over four decades the phenomena have been associated with different ranges of stimulus frequency. From 0.1 to 1 Hz the visual system dominates. in the range 1 to 10 Hz the LVOR phenomena dominate, in the range 20 to 200 Hz the low-frequency vibration OVEMPs are observed, which are likely utricular in origin, and in the range 200 to 1,000 Hz the AC OVEMPs are observed, which are likely saccular in origin. The two outer ranges evoke fundamentally different sensory combinations, being visual/vestibular and auditory/vestibular, respectively. For the two inner ranges, however, it is tempting to consider them to be actually a single domain, the high-pass property of the LVOR perhaps corresponding to the low-frequency tail of a band-pass sensitivity to head acceleration, with the OVEMP representing a high-frequency manifestation. In addition to the possibility of sharing a single band-pass sensitivity, both LVOR and vibration OVEMP phenomena share the property of being strongly modulated by eye gaze eccentricity (5, 18) and this further strengthens the view that they are two manifestations of the same physiological system, the otolith-ocular projections.
Aims.
The aims of the work reported here are to test the hypothesis that OVEMPs produced by whole head acceleration are a high-frequency manifestation of the otolith sensitivity underlying the LVOR. The basic design of the experiment was to subject human volunteers to NO-axis acceleration over a range of frequencies spanning 0.5 to 64 Hz at fixed peak acceleration of 1.0 ms−2 (i.e., 0.1 g). Directing the acceleration parallel to gravitational acceleration, by having the subjects supine, would ensure that there was no tilt component and that the acceleration was purely translational.
In the lower end of the frequency range we expected to observe in the POPs measured from both montages a significant EOG signal associated with eye movements, especially for earth-fixed conditions, which would conform to established properties, i.e., would be suppressed at 0.5 Hz by the VVI for head-fixed viewing, but would show low-pass sensitivity as a function of frequency. At the higher end of the range we expected to observe in the POPs measured from both montages a predominantly OVEMP signal associated with extraocular muscle activity, particularly at the highest frequencies as these approach the established best frequency of 80 to 100 Hz. At transitional frequencies we expected to observe in the POPs from both montages a mix of both EOG and OVEMP signals, which might combine in a nonlinear manner. We also anticipated the possibility of observing “presaccadic spike potentials” (17, 21) at the lower frequencies that are thought to be EMG signals associated with visually driven eye movements. Although these are particularly associated with saccadic eye movements, EMG activity preceding the low-frequency smooth-pursuit behavior during the VVI might also be present. If the OVEMP is a high-frequency manifestation of the LVOR then we should observe a high-pass effect in the POP gain of sensitivity as a function of gaze eccentricity that should be observable as a main effect of frequency in an ANOVA with POP gain as the dependent variable.
MATERIALS and METHODS
Subjects.
Six healthy male subjects with no history of vestibular or neurological impairment were recruited from the Institute of Sound and Vibration. All subjects had clear responses and because we had no prior data to carry out a power analysis, six subjects were considered sufficient for a first study. The experiment conformed to the Declaration of Helsinki and was approved by the Human Experimentation Safety and Ethics Committee of the Institute of Sound and Vibration Research and participants provided written informed consent.
Apparatus.
Whole body vibration was produced by a hydraulic vibrator at the Institute of Sound and Vibration. The vibrator was designed for research on human responses to vibration, including thresholds of vibration perception, vibration discomfort, physiological and performance effects of vibration and biodynamic studies (e.g., 9, 11). Within a 1-m vertical displacement it is capable of acceleration magnitudes up to about ±10 ms−2 with acceleration waveform distortion generally less than ∼10%. Drive waveforms for the vibrator were generated by CED Signal software in conjunction with a CED micro1401 DAC and presented to a Servotest Pulsar system that controlled the vibrator. Although primarily used for frequencies in the approximate range 0.1 to 50 Hz, the vibrator is capable of reproducing higher and lower frequencies. The highest stimulation frequency in this study was 64 Hz, so as to test at frequencies approaching the optimal stimulation frequency of the OVEMP. An accelerometer on the vibrator platform measured the acceleration produced by each stimulation sequence and verified that the required 1 ms−2 acceleration was achieved with good waveform at each frequency.
OVEMP and EOG montages were employed simultaneously to measure POPs with six Ag/AgCl electrodes above and below the eyes. For each eye, one inverting electrode was placed immediately inferior to the eye, one noninverting electrode ∼1 cm below the inverting electrode, and a second noninverting electrode above the eye. The two inferior electrodes constitute an OVEMP montage and the electrodes immediately below and above constitute an EOG montage, making four channels in total. POPs were amplified by means of a CED 1902 quad amplifier. To prevent biological amplifier saturation on the EOG channel, AC coupling was applied, equivalent to a high pass of 0.03 Hz. For the OVEMP channel a high-pass filter of ∼0.16 Hz was applied, as the differential montage was more susceptible to saturation. For all channels a low-pass filter of 1,000 Hz was applied. Raw and averaged POP data were sampled by a CED 1401+ at a sampling rate of 10,000 Hz. Although these settings are different from the standard for EOG, which usually employs a low pass of ∼20–30 Hz, and for the OVEMP montage, which usually employs a high pass of ∼5 Hz, it was important for our study to avoid loss of information or the introduction of distortion. The more open filtering was therefore appropriate.
Geometry of isovergence gaze targets.
Targets for eye gaze conditions were set on a frame that could be attached either to the vibrator or to the wall of the vibrator room. The targets were arranged to be isovergence (12) with a fixed viewing distance of 60 cm.
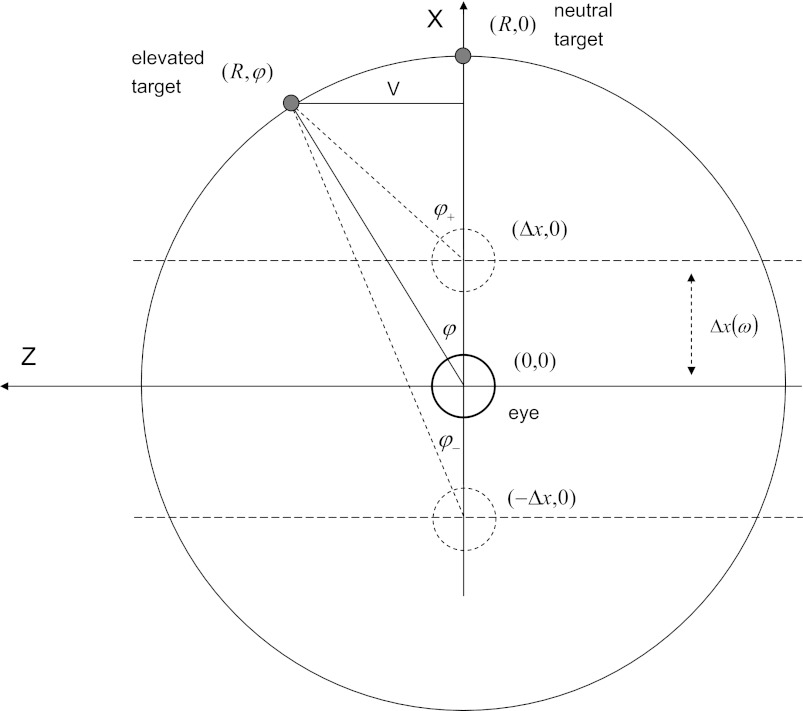
The geometry of the isovergence targets are illustrated in Fig. 1. In this arrangement the subject is supine so that with neutral gaze the line of sight is vertical and defined as the +ve x-direction. The +ve z-direction runs from left to right. If the viewing distance is R and the vertical eccentricity from neutral is V then prestimulus the resting gaze angle is given by sin φ = V/R, so that V = R sin φ. At the maximum +ve displacement Δx toward the target the required angle to maintain gaze is given by
and at the maximum −ve displacement away from the target the required angle to maintain gaze is given by
so that the total angular displacement for a full cycle is given by Δφ = φ+−φ−.
Fig. 1.
Geometry of the isovergence gaze targets. Subject is supine with head to the left. Eye positions relative to the targets are indicated by small circles. Dashed circles show eye positions at maximum displacement. Viewing distance is given by the radius R of the array. Vertical gaze eccentricity V is determined by the vertical resting gaze angle φ. Linear displacement Δx is a function of stimulus frequency.
Stimuli.
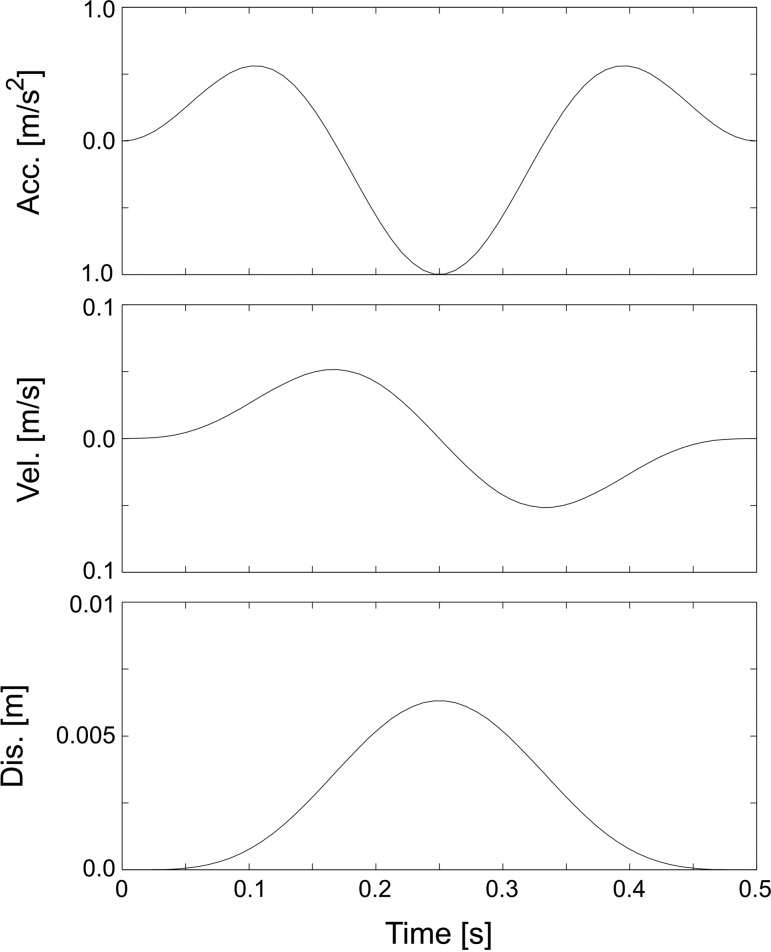
The stimuli were arranged over eight octaves from 0.5 to 64 Hz. The transient displacement stimulus waveforms consisted of cosine modulated ½ cycle (0.5 Hz), 1½ (1, 2, and 4 Hz), and 2½ cycle (8, 16, 32, and 64 Hz) sinusoids arranged so that at the endpoints of the motion the acceleration, the velocity and the displacement returned to zero (e.g., Fig. 2). The high-frequency stimuli were presented at a constant rate of about 1 per second while the low-frequency stimuli were presented at an interstimulus interval of about three cycle lengths. All stimuli were calibrated to have a peak acceleration of 1.0 ms−2 (i.e., 0.1 g). The calibration was performed during the iterative process for producing the drive waveforms by monitoring the shaker acceleration and adjusting the magnitude of the drive signal. The amplitude of the vertical displacement during a stimulus is approximated by Δx = a/ω2, where a is peak acceleration and ω is angular frequency, on the assumption that the motion was sinusoidal and of infinite duration, and values of these are shown in Table 1. From these data an ideal ocular angular displacement can be computed and an associated EOG value estimated using a typical calibration of 20 μV/°. In addition to POP recording, shaker acceleration and displacement waveforms were recorded in parallel from sensors on the vibrator table, and head acceleration was also recorded from a sensor attached to the subjects' forehead.
Fig. 2.
1½ cycle acceleration waveform and the corresponding velocity and displacements (example shown for 3-Hz fundamental frequency). [Reprinted from (11) with permission from Elsevier.]
Table 1.
Peak-peak platform vertical displacement, ocular angular displacement, and estimated EOG as a function of frequency (assuming a calibration of 20 μV/ °)
| f, Hz | ω, rad/s | ω2, rad/s2 | Δx, m | Δθ, ° | EOG, μV |
|---|---|---|---|---|---|
| 0.5 | 3.14 | 9.9 × 100 | 1.0 × 10−1 | 6.7 × 100 | 135 |
| 1 | 6.28 | 3.9 × 101 | 2.5 × 10−2 | 1.7 × 100 | 33.1 |
| 2 | 12.6 | 1.6 × 102 | 6.3 × 10−3 | 4.1 × 10−1 | 8.27 |
| 4 | 25.1 | 6.3 × 102 | 1.6 × 10−3 | 1.0 × 10−1 | 2.07 |
| 8 | 50.3 | 2.5 × 103 | 4.0 × 10−4 | 2.6 × 10−2 | 0.52 |
| 16 | 101 | 1.0 × 104 | 9.9 × 10−5 | 6.5 × 10−3 | 0.13 |
| 32 | 201 | 4.0 × 104 | 2.5 × 10−5 | 1.6 × 10−3 | 0.03 |
| 64 | 402 | 1.6 × 105 | 6.2 × 10−6 | 4.0 × 10−4 | 0.01 |
Δx, peak-peak platform vertical displacement; Δθ, ocular angular displacement; f, frequency; EOG, electrooculogram. Associated angular frequencies (ω) are shown. Values are computed for sinusoidal signals of infinite duration.
Procedure.
After the semi-supine subjects had been positioned on the vibrator table and restrained by a loose lap belt, the subjects were asked to remain relaxed but maintain their gaze on a target specified at the start of each recording run. For each of the stimulus frequencies, recordings of ocular responses were made in two contexts (head-fixed vs. earth-fixed), and with two gaze angles (0 and 20° up gaze) (i.e., 4 conditions for each frequency) to investigate the vestibular-visual interaction (VVI). The cycles of transient oscillation were repeated for between 30 and 80 trials with sufficient breaks to avoid subject fatigue. Because the low frequency stimuli had longer durations, fewer cycles were used with low frequency stimuli so as to minimize the duration of the experiment.
Data analysis and statistical methods.
All trials were first manually inspected at the level of POP traces to reject epochs with transient contaminating noiselike blinks or other sources of transient noise. Then all acceptable trials were averaged to obtain an individual average for each context (earth-fixed, head-fixed conditions), each montage (EOG, OVEMP), each frequency (from 0.5 to 64 Hz), each of the gaze conditions (0°, 20°) and each eye (left, right). Data analysis was carried out on each individual average for the POP magnitude (in μV), defined as the difference between the minimal and maximal peaks, and for the POP phase (in degrees), representing the POP response relative to the head acceleration. As the phase could shift throughout an epoch we chose to focus on the initial phase, defined by the first two cycles for the higher frequencies, and measured by means of cross-correlation. The POP response was considered as in-phase if the phase was close to 0° and the largest +ve peak in the cross-correlation used of the measurement and as anti-phase if the phase was close to 180° and the largest −ve peak used. On the basis of the POP magnitude averaging, a POP gain was calculated, defined as the ratio of POP magnitudes with the gaze at 20 and 0 deg.
Statistical analyses of the measurements were carried out in two combinations. In one combination, for the purpose of descriptive statistics from the marginal means, the earth-fixed and head-fixed conditions were analyzed separately. A 2 montages (EOG, OVEMP) × 8 frequencies (from 0.5 to 64 Hz) × 2 gazes (0°, 20°) × 2 eyes (left, right) ANOVA with repeated measures was applied both on magnitude and phase data. In a second combination, for the purpose of inferential statistics, low-frequency (from 0.5 to 4 Hz) and high-frequency (from 8 to 64 Hz) ranges were analyzed separately, but it should be noted that these divisions were chosen for the benefit of statistical convenience only as the transition in behavior occurs in the range 2–8 Hz. A 2 visual contexts (earth, head-fixed) × 4 frequencies (from 0.5 to 4 Hz or from 8 to 64 Hz) × 2 montages (EOG, OVEMP) × 2 gazes (0°, 20°) × 2 eyes (left, right) ANOVA with repeated measures was applied on both magnitude and phase data. A further analysis was carried out directly on the gain with a 2 visual contexts (earth, head-fixed) × 4 frequencies (from 0.5 to 4 Hz or from 8 to 64 Hz) × 2 montages (EOG, OVEMP) × 2 eyes (left, right) repeated-measures ANOVA. Possible departures from sphericity in combinations involving the frequency factor were catered for by using Greenhouse-Geisser estimates for significance levels. To take into account multiple comparisons, a 1% criteria was adopted as the critical level.
RESULTS
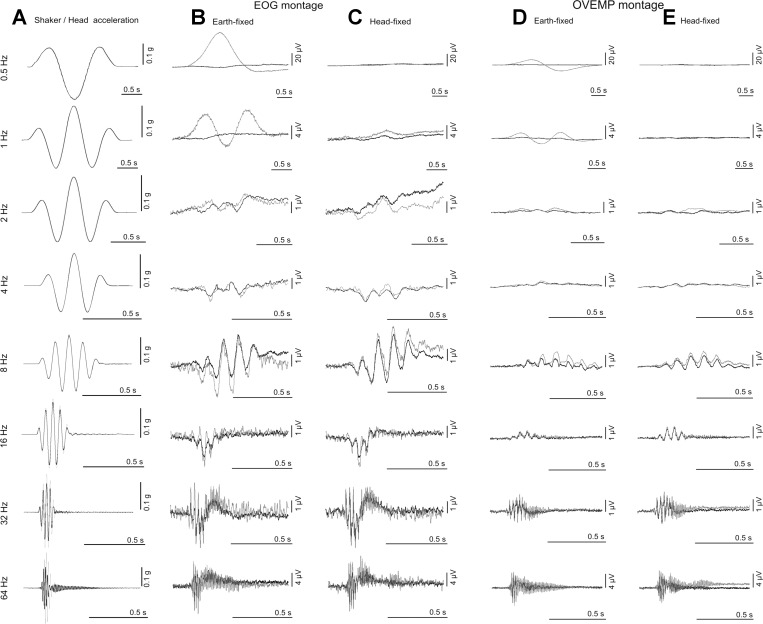
Grand means of the averaged recorded ocular responses to each of the four conditions for the eight stimulus frequencies are shown in Fig. 3. As shown in the leftmost column, the head acceleration closely follows the shaker acceleration up to 16 Hz in magnitude and phase. Above 16 Hz the phase of the head acceleration lags that of the shaker, most notably at 64 Hz. Head position follows a double integration of head acceleration, so with continuous sinusoidal acceleration a compensatory eye movement would be sinusoidal with 180° phase change relative to the acceleration. With transient motions, displacement waveforms differ from acceleration waveforms. With 1½ cycles of 0.5 Hz acceleration windowed as in this study, the displacement waveform was a single displacement pulse to and from one direction, giving rise to the unipolar signal from the EOG montage seen for the earth-fixed condition with 0.5 Hz oscillation in Figs. 3 and 4. At the high frequency end of the range the responses appear to be similar in the earth- vs. head-fixed conditions, whereas at the low-frequency end the responses show a marked difference between the earth- vs. head-fixed conditions. The high-pass filter of 0.16 Hz applied to the OVEMP channel resulted in differentiation of the 0.5 Hz responses.
Fig. 3.
Stimulus waveforms vs. response signals from electrooculogram (EOG) and ocular vestibular evoked myogenic potentials (OVEMP) montages for earth- vs. head-fixed conditions: A, shaker/head acceleration waveforms; B, signals from the EOG montage for the earth-fixed conditions; C, signals from the EOG montage for the head-fixed conditions; D, signals from the OVEMP montage for the earth-fixed condition; and E, signals from the OVEMP montage for the head-fixed conditions. For A the shaker acceleration is shown with the dark shading, whereas the head acceleration is shown with light shading. For B, C, D, and E the dark trace illustrates the 0° conditions and the light trace the 20° conditions.
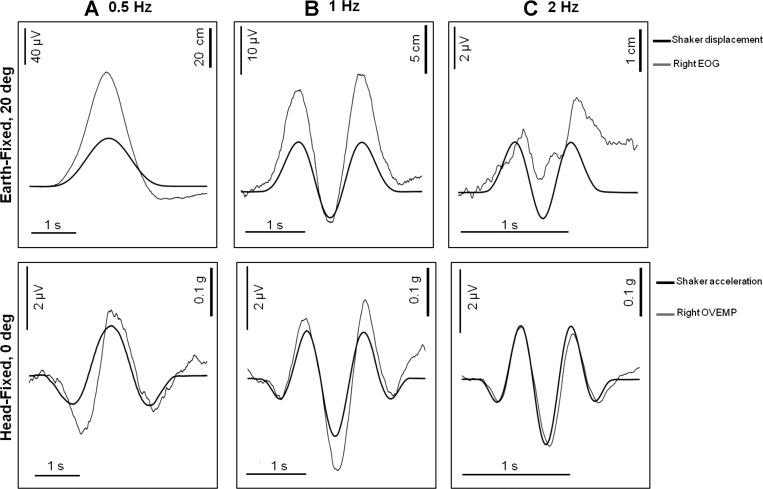
Fig. 4.
Periocular potentials (POPs) produced at low frequency to earth-fixed, 20° targets (top) and head-fixed, 0° targets (bottom): A, 0.5 Hz; B, 1 Hz; C, 2 Hz. For these frequencies there was a well-defined visual-vestibular interaction (VVI). Top shows signals from the EOG montage compared with shaker/head displacement (+ve up). Bottom shows signals from the OVEMP montage compared with shaker/head acceleration (−ve up).
To illustrate more closely the nature of the low-frequency responses, for which a VVI is apparent, we show in Fig. 4 responses from the 0.5, 1, and 2 Hz earth-fixed condition with gaze eccentricity of 20° (Fig. 4, top) compared with the responses for head-fixed with gaze eccentricity of 0° (Fig. 4, bottom). For the earth-fixed 20° condition the signal from EOG montage is closely correlated with the shaker and hence head displacement; with the signal from the OVEMP montage (not shown) dominated by this displacement following response. For 1 ms−2 at 20° eccentricity the angular displacement of the eye over a cycle of 0.5 Hz is 6.7°, which gives an EOG calibration of ∼17 μV/°g, which is in the normal range (∼20 μV per deg). The signal from the EOG montage drops off rapidly with frequency, as would be expected if the EOG signal is correlated with displacement (see Table 1). In contrast, for the head-fixed 0° condition, the signal from the OVEMP montage is closely correlated with the inverse of the head acceleration; the signal from the EOG montage (not shown) was dominated by this acceleration following response. The magnitude of the OVEMP does not change significantly over this range.
As previously explained, statistical analyses of the measurements were carried out in two combinations. In one combination, the earth-fixed and head-fixed conditions were analyzed separately. The marginal means from this combination are illustrated in Fig. 5, but with the head-fixed and earth-fixed conditions shown in the same panel for conciseness (numerical values of the means and standard deviations are provided in Tables 2 and 3). Viewing the overall pattern of magnitude of response as a function of frequency (Fig. 5, top) a well-defined U-shape curve is apparent. The largest responses are to be found at the low-frequency and high-frequency ends of the range and with smaller responses generally in the middle of the range, with the exception of 8 Hz where there is a well-defined peak against the general U-trend. The main contribution to the increase in magnitude at the low-frequency end of the U-curve is from the earth-fixed, 20° gaze condition where the signal from the EOG montage is largest. However, the other low-frequency conditions also show a “low-pass” trend, albeit to a lesser degree. At the high-frequency end of the U-curve, the largest contributions are from the 20° conditions where the OVEMP gaze effect is responsible. The “high-pass” trend is present in all of the conditions.
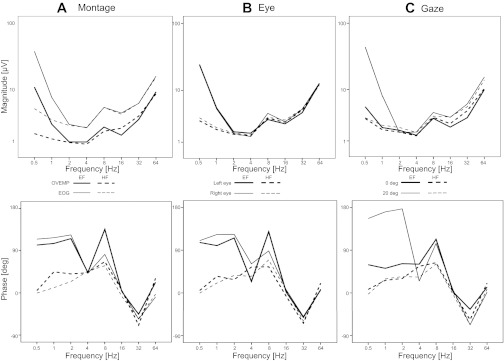
Fig. 5.
ANOVA marginal means of POP magnitude (top) and POP phase (bottom): A, effects of montage; B, effects of eye; C, effects of gaze. Magnitude data show a typical U-shape profile where the largest POPs are at the low and high-frequency ends of the range. Phase response shows distinct low- and high-frequency behaviors, where at the low-frequency end of the range there is a well-defined visual context effect.
Table 2.
Numerical values of ANOVA marginal means and standard errors for POP magnitudes as shown in Fig. 5, top
| Earth-Fixed |
||||||||
|---|---|---|---|---|---|---|---|---|
| Freq, Hz | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
| Montage | ||||||||
| OVEMP | 22.2 (3.2) | 5.0 (0.7) | 2.0 (0.3) | 1.9 (0.4) | 4.4 (1.3) | 2.9 (0.4) | 6.2 (1.1) | 18.6 (3.7) |
| EOG | 77.2 (8.1) | 15.1 (0.6) | 5.0 (0.2) | 4.3 (0.5) | 10.3 (1.1) | 8.2 (0.9) | 12.1 (1.3) | 32.8 (4.8) |
| Eye | ||||||||
| Left | 49.8 (5.8) | 10.3 (0.8) | 3.6 (0.3) | 3.4 (0.6) | 6.4 (0.7) | 5.3 (0.5) | 8.5 (1.2) | 25.5 (4.9) |
| Right | 49.6 (6.0) | 9.9 (0.6) | 3.3 (1.3) | 2.8 (0.4) | 8.3 (1.7) | 5.8 (0.5) | 9.8 (1.6) | 26.0 (5.2) |
| Gaze | ||||||||
| 0° | 10.3 (1.4) | 4.2 (0.4) | 3.6 (1.3) | 2.8 (0.4) | 6.4 (0.9) | 4.3 (0.4) | 6.6 (0.8) | 20.0 (3.2) |
| 20° | 89.0 (11.2) | 16.0 (1.0) | 3.3 (0.2) | 3.3 (0.4) | 8.3 (1.4) | 6.7 (0.7) | 11.6 (1.9) | 31.4 (5.1) |
| Head-Fixed |
||||||||
|---|---|---|---|---|---|---|---|---|
| Freq, Hz | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
| Montage | ||||||||
| OVEMP | 5.7 (2.7) | 4.6 (2.6) | 2.7 (1.1) | 1.7 (0.3) | 3.6 (0.6) | 4.2 (0.8) | 7.3 (2.0) | 19.6 (4.3) |
| EOG | 11.9 (2.5) | 8.9 (2.6) | 5.7 (0.9) | 4.4 (0.4) | 10.2 (1.3) | 7.8 (0.9) | 12.2 (2.3) | 33.4 (5.3) |
| Eye | ||||||||
| Left | 6.1 (0.4) | 4.0 (0.3) | 3.2 (0.3) | 2.9 (0.2) | 6.8 (0.9) | 5.7 (0.6) | 9.5 (1.9) | 27.5 (5.3) |
| Right | 11.6 (4.8) | 9.4 (5.1) | 5.2 (1.8) | 3.1 (0.3) | 7.0 (0.5) | 6.3 (0.6) | 10.1 (2.2) | 25.4 (4.3) |
| Gaze | ||||||||
| 0° | 8.7 (2.6) | 6.7 (3.1) | 4.8 (1.9) | 2.7 (0.3) | 6.7 (0.6) | 5.1 (0.2) | 8.9 (1.6) | 20.8 (4.0) |
| 20° | 9.0 (2.5) | 6.8 (2.1) | 3.6 (0.5) | 3.3 (0.3) | 7.0 (0.8) | 6.9 (0.5) | 10.7 (2.5) | 32.2 (5.3) |
Values are means (SE).
Table 3.
Numerical values of ANOVA marginal means and standard errors for POP phase as shown in Fig. 5, bottom
| Earth-Fixed |
||||||||
|---|---|---|---|---|---|---|---|---|
| Freq, Hz | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
| Montage | ||||||||
| OVEMP | 101.9 (13.9) | 105.3 (17.1) | 115.6 (39.8) | 43.5 (16.4) | 135.5 (19.0) | 3.4 (9.4) | −45.7 (13.5) | 22.5 (10.9) |
| EOG | 114.5 (19.7) | 117.5 (17.5) | 123.5 (20.8) | 42.1 (12.9) | 82.0 (16.5) | 5.6 (12.3) | −56.2 (8.5) | −8.6 (13.8) |
| Eye | ||||||||
| Left | 106.3 (16.1) | 99.7 (16.7) | 115.7 (26.9) | 24.2 (12.3) | 129.0 (20.8) | 2.1 (7.7) | −51.9 (6.8) | 6.4 (11.2) |
| Right | 110.1 (16.2) | 123.1 (20.6) | 123.4 (31.7) | 61.4 (28.2) | 88.4 (12.2) | 6.9 (5.5) | −50.0 (9.2) | 7.4 (13.3) |
| Gaze | ||||||||
| 0° | 59.5 (30.6) | 51.9 (29.9) | 61.8 (45.7) | 60.6 (15.4) | 113.2 (14.9) | 2.4 (10.7) | −34.6 (11.7) | 12.3 (13.7) |
| 20° | 156.9 (2.1) | 170.9 (3.0) | 177.2 (18.3) | 25.0 (8.6) | 104.3 (16.2) | 6.6 (6.5) | −67.3 (10.2) | 1.5 (10.5) |
| Head-Fixed |
||||||||
|---|---|---|---|---|---|---|---|---|
| Freq, Hz | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
| Montage | ||||||||
| OVEMP | 3.0 (14.9) | 37.4 (21.4) | 33.8 (11.2) | 43.0 (23.0) | 66.8 (26.5) | 6.1 (15.7) | −69.0 (12.6) | 31.2 (11.9) |
| EOG | −2.5 (11.6) | 6.2 (13.4) | 15.7 (22.1) | 46.6 (28.0) | 58.0 (15.0) | −7.1 (21.1) | −55.0 (6.6) | −2.6 (19.9) |
| Eye | ||||||||
| Left | 5.1 (10.8) | 35.2 (13.8) | 28.0 (18.3) | 54.5 (19.3) | 55.1 (18.1) | −6.8 (18.9) | −64.1 (9.4) | 20.5 (15.2) |
| Right | −4.6 (11.3) | 8.4 (15.0) | 21.5 (14.6) | 35.1 (9.9) | 69.7 (19.1) | 5.8 (18.1) | −59.9 (9.5) | 8.2 (17.3) |
| Gaze | ||||||||
| 0° | 2.4 (12.5) | 22.0 (21.6) | 15.1 (20.5) | 56.3 (17.6) | 63.8 (20.5) | 2.2 (15.4) | −56.6 (11.4) | 20.6 (17.3) |
| 20° | −2.0 (11.5) | 21.6 (17.5) | 34.4 (13.0) | 33.4 (15.6) | 61.0 (17.5) | −3.2 (24.3) | −67.4 (10.4) | 8.0 (15.0) |
Values are means (SE).
The overall pattern of phase relative to head acceleration (Fig. 5, bottom) is more complex than that of magnitude. The complexity of the phase response is added to by the fact that it shifts within a single response epoch at the higher frequencies and for this reason the phase is based on the first two cycles. Nevertheless, it is possible to distinguish different low-frequency and high-frequency behaviors. The largest effect in the low-frequency region is approaching a 180° phase difference between the earth-fixed 20° gaze condition and the head-fixed conditions. This effect corresponds to the displacement vs. acceleration following responses illustrated in Fig. 4. The head-fixed conditions show a general trend from in-phase with driving acceleration to a gradual increase in phase lag until 8 Hz where the lag is ∼90°. The low-frequency earth-fixed conditions are dominated by the effect of gaze. At the high-frequency end, the phase behavior is essentially identical across conditions. The phase returns to ∼0 at 16 Hz but then oscillates between leading and lagging at 32 Hz and 64 Hz. However, there is an inherent 360° ambiguity in the phase responses at these frequencies.
In the second statistical combination, the low-frequency (EOG dominant) and high-frequency (OVEMP dominant) ranges were analyzed separately. Considering first the magnitude data, there are highly significant main effects for all factors (P < 0.005) and all their interactions (P < 0.005) in the low-frequency domain, confirming the impression given in Fig. 4. In the high-frequency domain, however, the visual context effect (earth-fixed vs. head-fixed) drops out, leaving main effects of frequency (P < 0.01), gaze (P < 0.005), and montage (P < 0.005). Most of the interaction effects also drop out leaving interactions of frequency with montage (P < 0.01) and with gaze (P < 0.005). The frequency by gaze effect indicates that the change in magnitude with gaze is greater at higher frequencies.
For the phase in the low-frequency range there was a highly significant main effect of visual context (P < 0.005) indicating a large phase shift between the low-frequency earth-fixed vs. head-fixed conditions. Thus for the head-fixed conditions the responses essentially follow acceleration, whereas for the earth-fixed 20° gaze condition the responses follow displacement. The earth-fixed 0° gaze condition indicated an intermediate lag. Significant main effects are essentially absent for the other factors. For the earth-fixed conditions the phase lag is approximately constant and large for 0.5, 1, and 2 Hz, and jumping down to 45° at 4 Hz, whereas for the head-fixed conditions the phase lag is effectively 0 at 0.5 Hz, gradually increasing with frequency to ∼45 at 4 Hz. For the 0° gaze conditions the phase lag general increases from 0.5 to 4 Hz, whereas for the 20° gaze conditions the phase lag generally decreases. For the phase in the high-frequency range, the main effect of context drops out, but there is a main effect of frequency (P < 0.001). There are no significant interactions for phase at high frequencies.
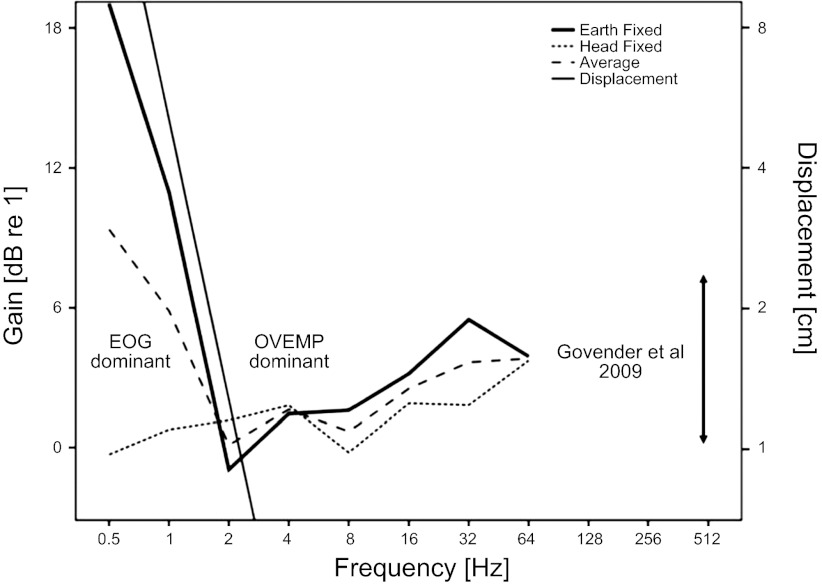
Given the complexity of the data and difficulty interpreting the POP magnitude interaction effects, a further analysis was carried out directly on the POP gain (i.e., on the POP magnitude ratio of 20° and 0° responses). The POP gains are illustrated over the whole frequency range in Fig. 6. It can be seen that the POP gain shows distinct low-frequency and high-frequency behaviors. From 0.5 to 2 Hz the POP gain for the earth-fixed response is strongly correlated in slope with the head displacement (on a log scale), whereas the head-fixed response shows an approximately unity gain. These responses are manifest in the ANOVA as main effects of context and frequency (P < 0.001) and an interaction between frequency and context (P < 0.005). In contrast, from 2 to 64 Hz the gain does not follow displacement in decreasing with frequency but is more acceleration-following in its behavior. However, even under the constant acceleration conditions of this experiment the gain increases with frequency (P < 0.01). The high-frequency patterns of significance in the ANOVA are similar to those of the low-frequency region, albeit with a smaller effect size. Thus there is also a high-frequency main effect of context in the responses (P < 0.01). To compare the high-frequency gain behavior with earlier literature Fig. 6 also indicates the range of gains at 20° found by Govender et al. (5), ∼1–8 dB.
Fig. 6.
Mean POP gains (ratio of POP magnitude at 20° and 0° gaze) as a function of frequency. Mean was computed from the average of EOG and OVEMP montage signals. POP gain shows distinct low- and high-frequency behavior. At frequencies <4 Hz the gain is correlated with displacement and decreases with frequency, i.e., is dominated by the EOG signal for the earth-fixed conditions. At frequencies >4 Hz the gain increases with increasing frequency and is dominated by the OVEMP response. Frequency range is extended to 512 Hz to illustrate the range of OVEMP gaze effect found in the experiment of Govender et al. (5) at 500 Hz.
DISCUSSION
The results show some clear trends. Reviewing the magnitude data shown in Fig. 5, at low frequencies a well-defined VVI is observed with response magnitude falling from 0.5 to 2 Hz, consistent with an EOG response, i.e., a retinal-corneal dipole displacement following head displacement, as illustrated in Fig. 4. Displacement-following responses are suppressed with a head-fixed target compared with an earth-fixed target as visual tracking is suppressed, and instead we observed acceleration-following responses in the signal from the OVEMP montage (Fig. 4). At higher frequencies the response magnitude increases with increasing frequency. This is not consistent with the response following displacement, because displacement reduces with increasing frequency if the acceleration is constant (as in Table 1). Rather, a high magnitude response is seen at frequencies greater than 4 Hz that increases with increasing frequency. This effect is consistent with the emergence of the high-frequency OVEMP response documented by Todd et al. (24) using mini-shaker stimulation. However, the mini-shaker was not capable of producing large displacements, so the previous work did not investigate the low frequency range used in the current study.
When results are analyzed in terms of eye position gain (defined as the ratio of POP magnitudes with the gaze at 20° and 0°), the low-frequency response for earth-fixed conditions correlates with head displacement, indicative that its primary source is a retinal-corneal dipole displacement, i.e., an EOG signal (Fig. 4). The POP gain also exhibits a high-pass effect, which cannot be explained by EOG, because the eye displacement, and hence expected EOG (Table 1), is too small and must therefore be myogenic in origin (i.e., an OVEMP response). Crucially for our hypothesis, the size of the high-frequency gain in our results is in the same range as found in previous studies (5), consistent with the view that the OVEMP as employed in clinical vestibular research is a high-frequency manifestation of the VOR.
Although the general trends of our data conformed to expectations, there were a number of unexpected results. There is a well-defined peak response at 8 Hz that went against the general trends, and we observed well-defined low-frequency acceleration-following responses, even in the head-fixed condition. Furthermore, although we expected and observed a VVI in the low-frequency end of the range, there was also evidence of a high-frequency visual context effect, consistent with a VOR interpretation.
Regarding the 8 Hz behavior in the data, this could be attributable to a nonlinear interaction of EOG and OVEMP or it may have had a biodynamic origin. There are resonances in and between various parts of the body at this frequency (9) so that whole-body vibration can give an enhanced response. Indeed, enhanced pitching motion of the heads of subjects was quite noticeable to the observer. However, the purpose of the experiment was to measure myogenic ocular responses with the head fixed relative to the platform. The apparent resonance at 8 Hz introduced an unexpected head pitching motion that will have provided additional vestibular stimulation and thus additional myogenic ocular responses. For this reason, the 8-Hz response should be treated with caution and its interpretation is not the main purpose of this paper.
The existence of acceleration-following responses at very low frequency for head-fixed and earth-fixed conditions with a zero-gaze eccentricity is surprising because we would expect that with complete visual suppression there should be no eye movement. It is, of course, possible that the VVI does not completely abolish compensatory eye movement, but in this case we would still expect the responses to follow the displacement, as is clearly the case for earth-fixed 20° gaze conditions where the phase lag is close to 180° relative to acceleration. In contrast, the head-fixed conditions have a phase-lag close to zero, i.e., they follow acceleration and are, therefore, fundamentally different from the responses associated with a compensatory eye movement.
Of particular importance to their interpretation is the observation that the sign of the head-fixed POP correlates with −ve acceleration (anti-phase with +ve acceleration). Thus, if the source of the signal in the OVEMP montage is myogenic, the +ve polarity corresponds to an inhibitory drive (the inverting electrode being closest to the muscles) and we must interpret the myogenic activity as opposing the acceleration in these cases, i.e., a +ve (upward) acceleration produces muscle inhibition. As such, this response might represent a form of low-frequency inhibitory OVEMP associated with the VVI. For the earth-fixed 20° condition we should in contrast expect an excitatory OVEMP, which would superimpose with the EOG signal. There is some evidence for this in the 2-Hz case shown in Fig. 4, where the magnitude of the EOG and OVEMP is similar. However, further work will be required to substantiate these interpretations.
Considering now the high-frequency responses, as noted in the introduction, it has been determined that the sensitivity of the LVOR to acceleration increases with increasing frequency, approaching, but not reaching, ideal sensitivity (compensatory angular eye movement corrects for displacement of the head exactly) at ∼10 Hz, e.g., McHenry and Angelaki (12). If our physiological data are a manifestation of the LVOR then we should expect to see this increase in gain up to 10 Hz, although previous studies have not gone to stimulation frequencies much higher than 10 Hz, so it is unclear what we should expect to happen to gain at higher frequencies. We observed that even at constant acceleration the magnitude of gain exhibits a high-pass behavior (∼0.5 dB per octave).
Some caution is required in interpreting these data because it is well established that the discharge pattern of extraocular eye motoneurons includes both velocity and position signals so that for saccadic eye movements the command signal has the form of a “pulse step” (10). For high-frequency oscillatory VOR movements, the command will be primarily a velocity signal. Furthermore, the appropriate motor command needs to compensate for the ocular plant dynamics, which will be frequency dependent (6, 20). Because the mechanical impedance of the eye-plant increases with increasing frequency, the motor command signal would need to increase accordingly so that even under constant velocity conditions we should expect that the EMG signal should show a “high-pass” response. Our observed physiological response may be a manifestation of both the high-pass property of the LVOR and of a requisite motor compensation for the low-pass ocular plant dynamics.
In addition to the expected effects, we also observed an additional effect of visual context. The mean earth-fixed response reaches a maximum gain of 5.5 dB at 32 Hz, whereas the mean head-fixed response continues to increase up to a maximum gain of 3.7 dB at 64 Hz. The existence of a difference in the earth-fixed vs. head-fixed conditions at high frequencies was not expected because the view from the literature is that the visual system has little influence at frequencies greater than ∼1 Hz. These differences in the earth-fixed vs. head-fixed responses may reflect the operation of two distinct mechanisms, a central mechanism that modulates the gain as a function of visual and eye position inputs (which could account for the extra earth-fixed gain up to 32 Hz; Fig. 6), and a second peripheral mechanism associated with the previously observed “resonance” at ∼100 Hz (which could account for a continued increase in gain beyond 32 Hz).
In the OVEMP literature it is generally thought that there are two possible mechanisms that contribute to the observed gaze effect. The first position is that the OVEMP is essentially analogous to the VEMP and modulated by background contraction (18). The second view is that gain in magnitude is attributable to the relative position of the inferior oblique muscle to the recording electrodes (2). Both these hypotheses, however, would imply that the gain as defined in our experiment should be independent of stimulus frequency, which is inconsistent with our data, and so an alternative explanation is necessary. One such alternative is that the change in magnitude with gaze is attributable to a central LVOR gain control mechanism that is influenced by both visual and eye-position inputs.
It is well established that the cerebellum plays a central role in the modulation of gain and phase of the VOR and of smooth pursuit eye movements (7). In the present situation, where the LVOR is mediated by a three-neuron arc through vestibular and oculomotor nuclei following afferent input from the otolith organs, central modulation is thought to be achieved primarily by the nodular and uvular divisions of the cerebellum (1, 29). The nodulus/uvula exert their influence by means of Purkinje cell inhibition on the vestibular nuclei but the strength of the influence can be modulated by retinal and eye proprioceptive inputs to the cerebellum via the inferior olive. In animal preparations cerebellar mechanisms may be investigated by inducing cerebellar lesions (29). For the human system, testing patients with cerebellar dysfunction may be a means by which the matter may further investigated.
Previously it has been speculated that the mechanisms that underlie the apparent 100-Hz “best response” to vibration in the OVEMP could be related to mechanical resonance in the otolith end organs and/or neural resonance of basolateral currents of otolith hair-cells (24). Having provided evidence that the OVEMP is a high-frequency manifestation of the LVOR we may suggest that the physiological function of the 100-Hz utricular resonance is to enhance the high-pass property of the LVOR and specifically the utriculoocular reflex in the critical range of stimulus frequencies >2 Hz. The presence of such a higher band pass is also implicit in early work by Fernandez and Goldberg (3) on the transfer function of otolith units, particularly the irregular units.
GRANTS
This work was supported by a grant from the Wellcome Trust (WT091961MA).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.P.M.T., S.L.B., and M.J.G. conception and design of research; N.P.M.T. and S.L.B. performed experiments; N.P.M.T., A.C.P., and M.J.G. analyzed data; N.P.M.T., S.L.B., A.C.P., and M.J.G. interpreted results of experiments; N.P.M.T. and A.C.P. prepared figures; N.P.M.T. drafted manuscript; N.P.M.T., S.L.B., A.C.P., and M.J.G. edited and revised manuscript; N.P.M.T., S.L.B., A.C.P., and M.J.G. approved final version of manuscript.
REFERENCES
- 1. Buttner-Ennever JA. A review of otolith pathways to brainstem and cerebellum. Ann NY Acad Sci 871: 51–64, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Curthoys IS, Vulovic V, Burgess AM, Cornell ED, Mezey LE, MacDougall HG, Manzari L, McGarvie LA. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function. Ann NY Acad Sci 1233: 231–241, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol 39: 985–995, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Ghasia FF, Meng H, Angelaki DE. Neural correlates of forward and inverse models for eye movements: evidence from three-dimensional kinematics. J Neurosci 28: 5082–5087, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Govender S, Rosengren SM, Colebatch JG. The effect of gaze direction on the ocular vestibular evoked myogenic potential produced by air-conducted sound. Clin Neurophysiol 120: 1386–1391, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Green AM, Meng H, Angelaki DE. A reevaluation of the inverse dynamic model for eye movements. J Neurosci 27: 1346–1355, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito M. Cerebellar control of the vestibular-ocular reflex—around the flocculus hypothesis. Annu Rev Neurosci 301: 275–296, 1982 [DOI] [PubMed] [Google Scholar]
- 8. Iwasaki S, McGarvie LA, Halmagyi GM, Burgess AM, Kim J, Colebatch JG, Curthoys IS. Head taps evoke a crossed vestibulo-ocular reflex. Neurology 68: 1227–1229, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Kitazaki S, Griffin MJ. A modal analysis of whole-body vertical vibration using a finite element model of the human body. J Sound Vib 200: 83–103, 1997 [Google Scholar]
- 10. Leigh RJ, Zee DS. Neurology of Eye Movements. Philadelphia: Davis, 1991 [Google Scholar]
- 11. Matsumoto Y, Griffin MJ. Effect of phase on human responses to vertical whole-body vibration and shock—analytical investigation. J Sound Vib 250: 813–834, 2002 [Google Scholar]
- 12. McHenry MQ, Angelaki DE. Primate translational vestibuloocular reflexes. II. Version and vergence responses to fore-aft motion. J Neurophysiol 83: 1648–1661, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Meng H, Angelaki DE. Neural correlates of the dependence of compensatory eye movements during translation on target distance and eccentricity. J Neurophysiol 95: 2530–2540, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Paige GD, Telford L, Seidman SH, Barnes GR. Human vestibuloocular reflex and its interactions with vision and fixation distance during linear and angular head movement. J Neurophysiol 80: 2391–2404, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol 65: 1170–1182, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. II. Visual-vestibular interactions and kinematic considerations. J Neurophysiol 65: 1183–1196, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Rodionov V, Elidan J, Sela M, Nitzan M, Sohmer H. Vertical plane short and middle latency vestibular evoked potentials in humans. Ann Otol Rhinol Laryngol 105: 43–48, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol 116: 1938–1948, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol 121: 636–651, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Sklavos S, Porrill J, Kaneko CR, Dean P. Evidence for wide range of time scales in oculomotor plant dynamics: implications for models of eye-movement control. Vision Res 45: 1525–1542, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thickbroom GW, Mastaglia FL. Presaccadic spike potential. Relation to eye movement direction. Electroencephalogr Clin Neurophysiol 64: 211–214, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Todd NP, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol 118: 381–390, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Todd NPM, Curthoys IS, Aw ST, Todd MJ, McGarvie LA, Rosengren SM, Colebatch JG, Halmagyi GM. Vestibular evoked ocular responses to air- (AC) and bone-conducted (BC) sound. I: Eye movements and timing in relation to vestibular evoked peri-ocular potentials (VEPP). J Vestib Res 14: 123–124, 2004 [Google Scholar]
- 24. Todd NPM, Rosengren SM, Colebatch JG. A utricular origin of frequency tuning to low-frequency vibration in the human vestibular system? Neurosci Lett 451: 175–180, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Todd NPM, Rosengren SM, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by impulsive translational acceleration. Clin Neurophysiol 119: 1638–1651, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Todd NPM, Rosengren SM, Colebatch JG. Tuning and sensitivity of the human vestibular system to low-frequency vibration. Neurosci Lett 444: 36–41, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Todd NPM, Rosengren SM, Colebatch JG. Vestibular evoked ocular responses to air- (AC) and bone-conducted (BC) sound. II: A neuroanatomical and physiological interpretation of AC-OVEMPs. J Vestib Res 14: 215–216, 2004 [Google Scholar]
- 28. Todd NPM, Rosengren SM, Colebatch JG. Vestibular evoked ocular responses to air- (AC) and bone-conducted (BC) sound. III: A neuroanatomical and physiological interpretation of BC-OVEMPs. J Vestib Res 14: 216–217, 2004 [Google Scholar]
- 29. Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. The cerebellar nodulus/uvula integrates otolith signals for the translational vestibulo-ocular reflex. PLos One 5: e13981, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber KP, Rosengren SM, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol 590: 3091–3101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welgampola MS, Migliaccio AA, Myrie OA, Minor LB, Carey JP. The human sound- evoked vestibulo-ocular reflex and its electromyographic correlate. Clin Neurophysiol 120: 158–166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang AS, Govender S, Colebatch JG. Tuning of the ocular vestibular evoked myogenic potential (OVEMP) to bone-conducted (BC) sound stimulation. J Appl Physiol 112: 1279–1290, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Zhang AS, Govender S, Colebatch JG. Tuning of the ocular vestibular evoked myogenic potential to AC sound shows two separate peaks. Exp Brain Res 213: 111–116, 2011 [DOI] [PubMed] [Google Scholar]