Summary
Background
Large leg ulcers (LLU) may complicate autoimmune diseases. They pose a therapeutic challenge and are often resistant to treatment. To report three cases of autoimmune diseases complicated with LLU.
Case Report
Case 1. A 55-year old woman presented with long-standing painful LLU due to mixed connective tissue disease (MCTD). Biopsy from the ulcer edge showed small vessel vasculitis. IV methylprednisolone (MethP) 1 G/day, prednisolone (PR) 1mg/kg, monthly IV cyclophosphamide (CYC), cyclosporine (CyA) 100mg/day, IVIG 125G, ciprofloxacin+IV Iloprost+enoxaparin+aspirin (AAVAA), hyperbaric oxygen therapy (HO), maggot debridement and autologous skin transplantation were performed and the LLU healed. Case 2. A 45-year old women with MCTD developed multiple LLU’s with non-specific inflammation by biopsy. MethP, PR, hydroxychloroquine (HCQ), azathioprine (AZA), CYC, IVIG, AAVAA failed. Treatment for underlying the LLU tibial osteomyelitis and addition of CyA was followed by the LLU healing. Case 3. A 20-year-old man with history of polyarteritis nodosa (PAN) developed painful LLU’s due to small vessel vasculitis (biopsy). MethP, PR 1 mg/kg, CYC, CyA 100 mg/d, AAVAA failed. MRSA sepsis and relapse of systemic PAN developed. IV vancomycin, followed by ciprofloxacin, monthly IVIG (150 g/for 5 days) and infliximab (5 mg/kg) were instituted and the LLU’s healed.
Conclusions
LLU are extremely resistant to therapy. Combined use of multiple medications and services are needed for healing of LLU due to autoimmune diseases.
Keywords: leg ulcer, vasculitis, maggots, combined therapy
Bakcground
Large leg ulcers (LLU, surface area >100 cm2) are extremely difficult to heal [1]. Large leg ulcers have been previously described in patients with mixed connective tissue disease (MCTD) [2], scleroderma overlap syndrome (SOS), rheumatoid arthritis (RA) [3], Behcet diesease [4,5]. We report three cases of autoimmune diseases complicated with LLU resistant to regular wound therapy. The LLU healed only due to combined therapy contributed by multidisciplinary team.
Case Report
Case 1
A 55-year old woman was admitted to hospital because of appearance of multiple painful skin ulcerations on both shins during last two months followed by high fever (38.6°C) and massive discharges from the wounds with extended necrotic area (Figure 1A). The patient has had MCTD for twenty years presented with myositis, lupus-nephritis, and scleroderma. She was aggressively treated in her past with high doses of prednisone (PR) and cytotoxic drugs. She had high titers positive anti-nuclear (ANA) and anti-RNP antibodies (Ab). For the last ten years her disease was controlled with PR 10 mg/day and hydroxychloroquine (HCQ) 400 mg/day. Her medical history included severe destruction of both knees and hips as a consequence of multiple avascular bone necrosis; ten years before admission she underwent bilateral total hip replacement. On admission the patient looked very ill and disabled. She was febrile (38.6°C). On examination there were signs of sclerodactyly and typical for systemic sclerosis facial features. There were neither signs of active synovitis nor signs of internal organ involvement or damage. There was notable tenderness and movement limitation of let hip due to known prosthesis dysfuntion. There was left inguinal lymphadenopathy. Her peripheral pulses were palpable. There were multiple left leg deep shin ulcers, one of them was circularly huge with wide necrotic areas and purulent discharge. Laboratory tests showed: normocytic anemia, hypoalbuminemia, elevated CRP and accelerated ESR, mildly elevated liver enzymes. Tests for ds DNA, anti-Smith, anti-SSA, anti-SSB, anti-Scl70, anticentromere, ANCA, cryoglobulines, cardiolipin (ACL), b2-glycoprotein Ab, lupus anticoagulant (LAC), HBSAg and anti-HCV Ab were negative. Blood and urinary cultures were negative. Repeated wound cultures showed a range of pathogens: Ps. Aeroginosa, Cinetobacter baumannii, Citrobacter Freundii, E. fecalis, Stenotrophomas maltophilia, Serratia Marcescens. Their antibioticogramms were analyzed.
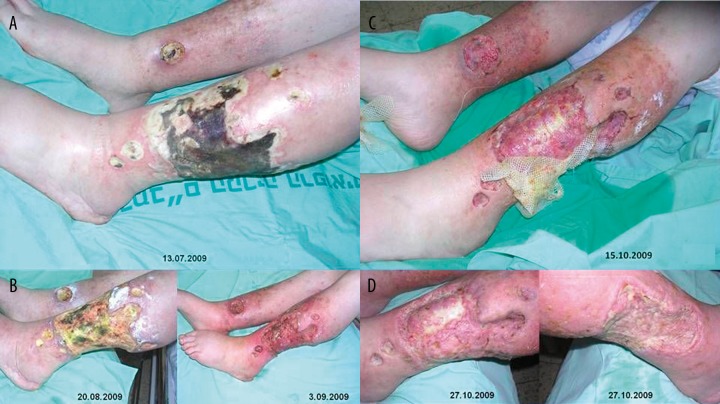
Figure 1.
Stages of the large ulcer healing are seen: necrotic ulcer on admission (A), good cleaning after maggot debridement (MDT) ((B) – left before and right after MDT), the wound 10 days after skin transplantation (application of local dressing is seen) (C), the ulcer is healing (good “take”) 22 days after skin transplantation (D) (Case 1).
Biopsy from ulcer edge showed small vessel vasculitis (Figure 5). The working diagnosis of vasculitis due to MCTD was made. The patient was still febrile, several episodes of atrial fibrillation developed (one of them was followed by severe dyspnea), and her LLU continued to spread with additional necrotic areas. Normal and negative blood cultures echocardiography excluded endocarditis. On chest and abdominal computed tomography (CT) small areas of bibasilar lung fibrosis were found, large multiple angimyolipomas were found in liver and both kidney (the relevance of this finding for patient’s condition remained unexplained). At that point united team of rheumatologist, dermatologist, plastic surgeon, physician of hyperbaric oxygenation service, orthopedic surgeon, specialist in imaging developed a therapeutic agenda, which included:
Figure 5.
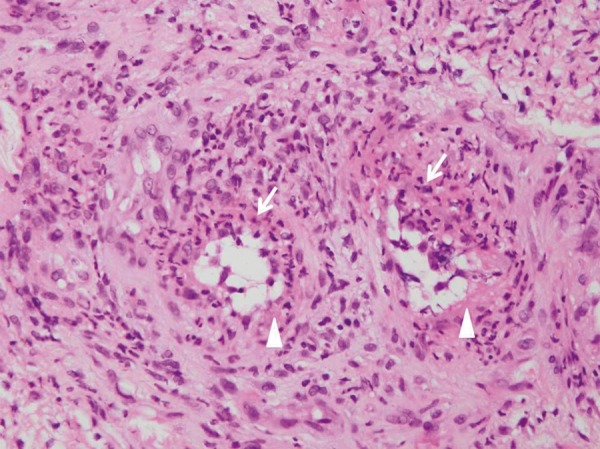
Two small blood vessels in the dermis underlying the ulcer showing fibrin deposits (arrow head) in the thickened blood vessel walls with intimal proliferation, neutrophils (arrows) and nuclear dust. These finding are compatible with acute small vessel vasculitis due to MCTD (Hematoxylin and eosin, ×400) (Case 1).
-
Therapy of background disease in order to achieve full remission of vasculitis
A – Methylprednisolone (MethP) pulses 1 g/day for 3 days, followed by daily MethP 100 mg intravenous (IV) infusions for two weeks, with further dose reduction to PR 1 mg/kg/day. Every two weeks the dose was diminished by 10% until achieved 20 mg PR/day, which was given up to full LLU repair.
B – IV CYC 0.75 g/m2 by monthly pulses. The therapy was interrupted because of active infection (thigh abscess) and event of pulmonary congestion followed the second IV infusion.
C – Cyclosporine A (CyA) 100 mg/day under blood level control till full LLU repair. The medication was stopped transiently during active infection (thigh abscess) and before skin grafting.
D – Intravenous immunoglobulines 30 g/per day for 5 days one course. Because of infusion reaction IVIG were stopped.
-
AAVAA complex [2]
A – Antiseptic medication for local use. Polydine applications was not tolerable and followed by application with Paranet (Vernoncarus, UK), Aquacell, Polymem, Arthroman and Tenderwet dressing.
A – Long-term Antibiotic IV therapy according to sensitivity of the ulcer flora till full repair of LLU [Amoxycillin+Clavulanic acid (Amoxy/Clav), Ciprofloxacin, Ceftriaxone]. Later Ciprofloxacin oral long-term therapy was introduced as regeneration activator (500 mg twice a day).
V – Vascular medication: prostaglandin Iloprost IV 50 mcg/day for at least 3 weeks.
A – Anticoagulant therapy with subcutaneous (SC) injections.of low molecular heparin Enoxaparin 1 mg/kg/day.
A – Antiplatelet therapy with low dose Aspirin 100 mg/day.
-
Hypebaric oxygenation therapy (HBOT)
Before hyperbaric oxygenation therapy pO2 of the wound tissue was tested and showed very low value (12 mmHg, N >30 mmHg). After 100% oxygen therapy the patient’s tissue pO2 increased to 288 mmHg, and after an increase of pressure to hyperbaric level of 2.4 ATA the tissue oxygenation raised to 675 mmHg that directly indicated the need for hyperbaric therapy. The patient underwent 8 sessions of the HBOT, each treatment was for one hour at 2.4 ATA and 100% oxygen.
-
Maggot debridement therapy (MDT)
The patient underwent two 48 hours courses of the MDT (Figures 1B, Figure 2). The MDT was followed by intractable pain, which stopped after removing of the maggots. A huge thigh abscess developed in the ill leg 2.5 weeks after the first maggot course. It is unclear, if there is any relationship to the MDT. The complication was successfully treated with open drainage, IV-broad spectrum antibiotic followed by prolonged oral ciprofloxacin (1 G/daily) according to sensitivity of Pseudomonas aerugenosa.
Skin graft. Following HBOT and second MDT course, and under therapy with AAVAA-complex partial thickness skin graft was harvested from the same leg (thigh region). The skin was meshed in 1:1.5 ratio and covered the wound. The “take” of the skin was good. Donor site was healed three weeks post operatively (Figure 1C,D).
Figure 2.
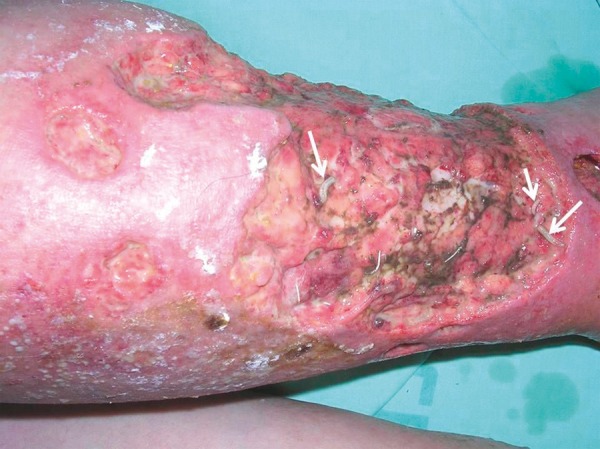
Maggots of the green bottle fly Lucilia (Phacnicia) scricata (arrows) are seen in the wound after maggot debridement. Most of the larves have already been removed after good cleaning of the wound (Case 1).
The patient had sixteen weeks of hospitalization that was complicated by episodes of atrial fibrillation, pulmonary congestion, and thigh abscess with surgical drainage. We used stepped approach: after inadequate response to 1 and 2 treatment modalities, we used 3rd and 4th, and finally 5th (skin graft). Simultaneous use of all modalities should be considered as alternative in order to condense recovery period. The patient was dicharged home while being on PR 10 mg/day, HCQ 400mg/day, CyA 100 mg/day, aspirin 100 mg/d, vitamin D and Calcium supplementation.
Case 2
A 45 year old women was admitted with a history of painful LLU for 3 months (Figure 3A). She had MCTD (rheumatoid arthritis, lupus nephritis, pneumonitis) since 1988 and was treated with PR 15–60 mg/day, azathioprine (AZA) 150 mg/day, HCQ 400 mg/day for last several years. At the time of the LLU appearance her MCTD presented as non-active. Peripheral pulses were normal. Her blood tests were unremarkable except raised sedimentation rate and positive anti-RNP Ab. The skin ulcer biopsy was not conclusive for vasculitis and showed diffuse inflammation with granulation tissue. Combined therapy was administered and comprised of six pulses intravenous cyclophosphamide 1g/month instead of AZA, daily PR 1 mg/kg, intravenous Iloprost, Aspirin, IVIG (125G for 5 days), repeated courses of antibiotic therapy according to sensitivity of the wound pathogens and local therapy with applications of Aquacell (hydrocolloid fibres of sodium carboxymethylcellulose). Despite such aggressive treatment her LLU persisted. Tibial osteomyelitis was found by bone scintigraphy. Deep bacterial specimen revealed Bacteroides and Proteus mirabilis growth sensitive to Amoxy/Clav. Surgical debridement and three month Amoxy/Clav therapy with addition of of CyA 150mg/day and SC injections of Enoxaparin 40 U/day brought to complete LLU healing (Figure 3B).
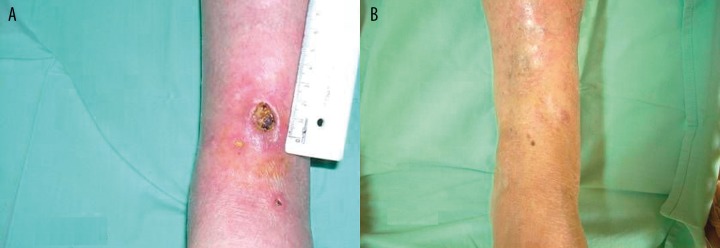
Figure 3.
Several ulcers are seen in patient with MCTD and underlying tibial osteomyelitis (A), successfully treated with surgical debridgement and long-term antibiotic therapy (Amoxy/Clav) in addition to immunosuppresors, corticosteroids and the AAVAA complex (B) (Case 2).
Case 3
A 20-year-old young man was admitted with history of recurrent painful red indurations of both shins for 5 year. These skin lesions deteriorated in past half year with appearance of livedo reticularis and very painful symmetric LLU (Figure 4A). At age 3 years the patient was diagnosed with severe polyarteritis nodosa (PAN) presented with high fever, skin rash, ocular palsy, acute intestinal ischemia and perforation. He was treated that time with high doses of steroids, IV CYC, and achieved long term drug-free remission. On admission no internal or neurological involvement was revealed. His blood pressure was normal. Peripheral pulses were palpable. His laboratory data showed: leucocytosis, normocytic anemia, mildly elevated liver ensymes, lower borderline albumin level, highly elevated CRP and accelerated ESR and normal kidney function, urinary analysis, and daily urinary protein. HBSAg and HCVAb were negative. Blood cultures were sterile. Wound cultures showed St. aureus. Screening for RF, ANCA, ANA, cryoglobulines, ACL, LAC, angiotensin-converting enzyme, viral and bacterial serology were negative. Chest and abdomen CT imaging was unremarkable. Skin biopsy was compatible with small vessel vasculitis. Treatment schedule included: MethP three 1 g/day pulses followed by PR 1mg/kg, two CYC IV pulse therapy (1.5 g/pulse) AAVAA complex [2], IVIG 30 g/day for 5 days (Table 1). The course of the disease was complicated with febrile episode with positive MRSA wound cultures but negative blood cultures that was treated with IV Vancomycine for two weeks followed by prolonged high dose oral Ciprofloxacine (1500 mg/day) treatment. Early improvement was registered after 17 days of the therapy. His disease relapsed after reducing of PR and discontinuation of CYC and IVIG infusions. That time along with his painful LLU he had also severe peripheral sensor polyneuropathy with left hand paresis. CyA was introduced but stopped due to vomiting. His PR doses were raised again up to 1 mg/kg/day, additional 6 IV CYC pulses (1.5 g per/month), IVIG 125 g/5 days/month was reinstituted, and IV infusions of Infliximab 5 mg/kg every 8 weeks (after first 3 infusions on weeks 0, 2, and 4) was added. Under this therapy gradual improvement in patient general condition, and neurological status has been achieved followed by complete LLU repair after 246 days (Figure 4B).
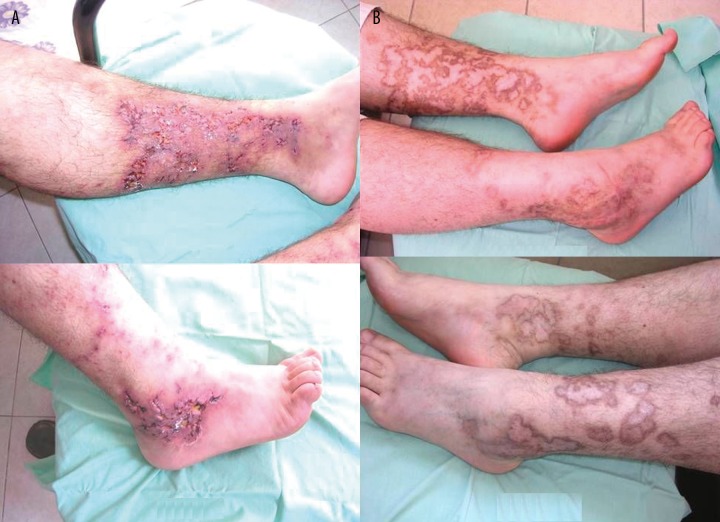
Figure 4.
Symmetric LLU’s and livedo reticularis are seen in patient with active polyarteritis nodosa (A). (B) showes hyperpigmentation and complete healing of LLU’s after 246 days of combined therapy (Case 3).
Table 1.
Dynamic of the skin ulcer state according to received therapy (Case 3).
| Data | 29.08.06 | 6.9.06 | 19.9.06 | 28.9.06 | 14.11.06 | 30.11.06 | 10.1.07 | 1.2.07 | 17.4.07 |
|---|---|---|---|---|---|---|---|---|---|
| Day of therapy | 17 | 25 | 38 | 47 | 92 | 108 | 148 | 169 | 246 |
| Therapy | AAVAA+ Cyc+PSMP IVIG+PR | AAVAA +PR | AAVAA+Cyc +PSMP IVIG +CyA+PR | AAVAA +CyA+PR | Cyc +PSMP +CyA +PR | Cyc +PSMP +CyA +PR | AAVAA +Cyc +PSMP IVIG +Rem PR | AAVAA +Cyc +PSMP IVIG +Rem PR | AAVAA +Cyc +PSMP IVIG +Rem +PR |
| State of skin ulcer | Start of gran, crusts, no discharge | Start of gran, crusts, no discharge | Gran, crusts, partial ulcer healing | Gran, crusts, partial ulcer healing | Complete ulcer healing | Complete ulcer healing | Ulcer relapse Neuropathy | Ulcer gran, crusts | Ulcer healing, hyperpygmentation |
AAVAA – aseptic, antibiotic, vascular, anticoagulant, antiaggregant complex; Cyc – cyclophosphamide; PSMP – puls methylprednisolone; IVIG – intravenous immunoglobulines; Rem – Remicade (Infliximab); PR – prednisone; gran – granulations.
Discussion
The understanding of the mechanisms that act to inhibit and delay large long-standing wound healing becomes a true science rather than a clinical art [6]. Vascular, infectious and tumor-associated causes, pyoderma gangrenosum and pressure sores must be considered in patients with spread skin ulcerations and CTD. Ulcerations secondary to ischemia may accompany severe secondary to CTD Raynaud’s phenomenon, vascular thrombosis secondary to coagulation abnormalities (presence of prothrombotic factors or antiphospholipid syndrome), high viscosity due to dysproteinemia, abnormal vascular supply secondary to inflammation or necrosis of vascular wall (necrotizing venulitis or arteritis). Almost all listed factors contributed to development of LLU in presented here patients. MCTD (case 1 and 2) has always been considered as a benign rheumatic condition with a favorable response to therapy. Serious vascular complications with thrombotic events, pulmonary hypertension, heart failure, severe Raynaud’s phenomenon and often ischemic skin ulcers are common in MCTD; they often result in significant morbidity and mortality [2,7]. In presented here two cases of MCTD rare vascular complication – skin vasculitis with spread and deep skin necrosis – resulted in a recalcitrant LLU with severe infective complications (huge thigh abscess in Case 1 and osteomyelitis in Case 2) resistant to routine treatment. In Case 3 LLU preceded relapse of PAN, they did not responded to conventional treatment and like in first two cases were complicated by MRSA colonization in the wounds. All three patients needed prolonged, complex and combined therapy that could be possible only by multidisciplinary teem recruitment. The rationale for this complex therapy for the leg ulcers was as follows.
The necessary constituent of successful treatment was aggressive immunosupressive therapy for vasculitis with CYC and MethP pulse therapy, followed high PR doses, and addition of CyA. Obvious control of basic CTD in case 1 and case 2, did not result in LLU healing. In case 3 partial control of systemic features in the course of PAN with IVIG, steroids and CYC did not result in LLU healing either. In this patient only addition of anti TNF agent (Infliximab) allow to control both – systemic appearance and skin necrosis.
It is obvious that there is a need to treat local infection with effective local antibiotic long-term therapy according to microbial susceptibility up to complete wound healing. Wound infection often are responsible for the unhealed ulcers and be an origin for microbial dissemination through this “open gate” as it caused in all our patients. Underlying occult and deep infection (underlying osteomyelitis in case 2) by contiguous or remote mechanism should be suggested in cases of deep ulcers resistant to intensive treatment. Careful search using different diagnostic tools is advocated in these cases. Local dressingsallows control and eradication of secondary infections, which may be present in the lesion, and at the same time provides an excellent mechanical protection which does not adhere to granulating skin. Application of the dressing does not result in maceration of the lesion and in experienced hands may be removed painlessly. The role of systemic antibiotics is heard to overwhelm. In all presented cases severe local infections responded only to intravenous broad-spectrum antibacterial therapy. In two cases IV antibiotics were successfully switched to high doses long-term oral ciprofloxacin.
Besides its antibiotic properties, ciprofloxacin was reported as an activator of interleukin-3 production, granulocyte-macrophage colony-stimulating factor and haemopoesis [8–11], which are important for wound healing (tissue regenerator). It also considered as an inhibitor of antiphospholipid antibodies [12]. A high dose of ciprofloxacine has been reported to inhibit TNF-a production, lymphocyte blast transformation and synthesis of immunoglobulins [13,14]. We assesses ciprofloxacin as crucial component of combined therapy for tissue regeneration. As in previous report [2] we observed three-phasic regeneration in wound healing. The first initial phase is very slow (first month) as is associated with treatment of the affecting factors interfering with normal regeneration: infection, inflammation, vascular damage and thrombosis. The second period (1–2 months) was associated with rapid wound regeneration, which was followed by the third period (after two months) of slower healing due to phenomenon of contact cell inhibition. These periods may be much more long due to active not neutralized affecting factors.
MCTD and PAN are characterized by microvascular impairments of small and medium-sized vessels presented as intimal proliferation (Figure 5), medial hypertrophy, intravascular thromb formation and occlusion [15] accompanied by hypercoagulation tendency (high titers of D-dimers, high factor VIII, elevated fibrinogen level, high titers of anticardiolipin antibodies) [2]. Reported high prevalence of medium-sized vessel occlusions in patients with MCTD may need angiographic studies [16]. Prominent vascular changes justifie aggressive therapy with anticoagulants, aspirin and Iloprost. The anticoagulation therapy has been reported to be beneficial in MCTD associated with leg ulcer [2], arterial thrombosis [17], pulmonary hypertension [18], Budd-Chiari syndrome [19], and pulmonary thromboembolism [20]. A number of double-blind placebo-controlled studies of IV Iloprost infusion have shown benefit in Raynaud’s syndrome and digital ulcers healing [21,22]. In addition to its vasodilatory and antiplatelet effects, Iloprost has been shown to down regulate lymphocyte adhesion to the endothelium [23]. Iloprost was shown to restore the innate anti-oxidant system in patients with Raynaud’s phenomenon secondary to systemic sclerosis [24]. Iloprost is the first choice for patients with critical digital ischaemia or ulceration. It can produce benefit lasting for between six weeks and six months in most patients.
MDT was first introduced in America in 1931. Sterile maggots of the green bottle fly Lucilia (Phacnicia) scricata are used for MDT. Up to 1000 maggots are introduced into the wound and left for 1–3 days. One of the major advantages of MDT is that the maggots separate the necrotic tissue from the living tissue. In 80–95% of cases a complete or significant debridement of the wound is achieved. An immediate amputation can be prevented as a result of MDT [25]. In another study 21 ambulatory patients with non-healing ulcers were treated with MDT [26]. It is reported as a simple, efficient, well tolerated, and cost effective tool for the treatment of wounds and ulcers that do not response to conventional treatment [25]. We suggest that as a tool for effective debredement, MDT should be used as first line treatment along with systemic management in order to achieve a rapid and complete effect [3]. Our experience with MDT showed their highly effective cleaning effect (Figure 1B) that may be accompanied by severe exacerbation of wound pain (case 1). The exacerbation of the wound pain during MDT is a known side effect.
IVIG are current used in autoimmunity. Generated from plasma of more than 10000 healthy subjects and originally employed to supplement the missing immunoglobulins in subjects with immune-deficiencies it was given successfully to patients with different CTD such as polymyositis, systemic lupus erythematosus, antiphospholipid syndrome and etc. The mechanisms by which IVIG exerts its beneficial effects in autoimmune conditions were found and include anti-idiotypic activity against pathogenic autoantibodies, induction of antigen tolerance, antigen neutralization, immunomodulaion and etc [27]. First report of successful therapy for leg ulcer with IVIG was published in 1998 [28]. Since IVIG was widely used for therapy of refractory leg ulcers due to pyoderma gangrenosum [28,29], RA [30], livedoid vasculitis [31], periarteritis nodosa [32,33], chronic leucocytoclastic vasculitis [34]. We used schedule 0.4 g/kg/day for 5 days every month [27], which proved to be successful. We see a crucial role of IVIG in case 3, patient with PAN, which developed relapse after 2 months delay of the next IVIG course.
Tissue hypoxia is the key feature in many patients with non-healing wounds. Therefore the marked increase in oxygen tension gradient from the blood to metabolizing cell while breathing 100% oxygen at 2 bars in a hyperbaric chamber (2 atmospheres absolute) is the major mechanism by which hyperoxygenation can improve effective cellular oxygenation even at low rate of tissue perfusion.
Several randomized controlled clinical trials have studied HBOT for the treatment of chronic wounds [35]. Multiple components of the wound healing process are turned on by oxygen gradient. That explains why HBOT can be an effective therapy to treat chronic wounds. Angiogenesis, fibroblast proliferation and collagen synthesis are oxygen dependent [36,37]. Hyperbaric oxygen also has been shown to have direct and indirect antimicrobial activity, including re-establishment of defense mechanisms by the leukocytes. The rate of production of any oxygenases will then be raised. Another important effect of hyperoxia that is often overlooked is its ability to harness and control an exaggerated inflammatory response that is frequently observed in non-healing wounds [38]. In case 1, HBOT was one of the modalities in the complex treatment of LLU.
Split-thickness skin grafts can tolerate less ideal conditions for survival, for example the condition of chronic wound such as in case 1, and have a much broader range of application compared to full thickness skin graft. The ultimate success of a skin graft, or its “take”, depends on nutrient uptake and vascular in-growth from the recipient bed, for this reason the procedure was done in case 1, as in other cases of chronic wounds, only after achieving a good granulation tissue that covers the wound bed. Split-thickness skin graft donor sites heal spontaneously with cells supplied by the remaining epidermal appendages.
Conclusions
In conclusion, LLU due to CTD are extremely resistant to accepted conventional treatment. They often are complicated with severe local (osteomyelitis, abscess) or general (sepsis) infections that should be treated aggressively broad-spectrum antibiotics for a long time. Combined approach precipitates early diagnosis and appropriate treatment: effective use of immunosuppression, vasodilators, anticoagulants, antiaggregants, wound debridement (surgical or biological), hyperbaric oxygenation, the use of regeneration activators (ciprofloxacin), and skin transplantation. Patient’s and medical team patience will be appreciated when these hopeless and debilitating conditions will be cared by combined and persistent efforts of multidisciplinary team.
Footnotes
Declare no conflicts of interest
Source of support: Departmental sources
References
- 1.Balaji P, Mosley JG. Evaluation of vascular and metabolic deficiency in patients with large leg ulcers. Ann R Coll Surg Engl. 1995;77:270–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Rozin AP, Braun-Moscovici Y, Bergman R, Balbir-Gurman A. Recalcitrant leg ulcer due to mixed connective tissue disease. Nether J Med. 2005;64:91–94. [PubMed] [Google Scholar]
- 3.Rozin AP, Balbir-Gurman A, Gilead L, Slodownik D. Combined therapy for pyoderma gangrenosum. Ann Rheum Dis. 2004;63:888–89. doi: 10.1136/ard.2003.016824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi A, Hashimoto T. Oral prostaglandin E1 as a therapeutic modality for leg ulcers in Behcet’s disease. Int J Clin Pharmacol Res. 1987;7(4):283–89. [PubMed] [Google Scholar]
- 5.Erer D, Gogus F, Tumer NB, et al. Prevalence of Behcet’s syndrome in patients presenting with venous thrombosis: prospective study in a cardiovascular outpatient clinic. Arch Med Sci. 2009;5(3):371–75. [Google Scholar]
- 6.Clark M, Price PE. Is wound healing a true science or a clinical art? Lancet. 2004;364:1388–89. doi: 10.1016/S0140-6736(04)17240-1. [DOI] [PubMed] [Google Scholar]
- 7.Burdt MA, Hoffman RW, Deutscher SL, et al. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum. 1999;42:899–909. doi: 10.1002/1529-0131(199905)42:5<899::AID-ANR8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Shalit I. Immunological aspects of new quinolones. Eur J Clin Microbiol Infect Dis. 1991;10:262–66. doi: 10.1007/BF01966999. [DOI] [PubMed] [Google Scholar]
- 9.Kletter Y, Riklis I, Shalit, et al. Enhanced repopulation of murine hematopoetic organs in sublethally irradiated mice after treatment with ciprofloxacine. Blood. 1991;75:1685–91. [PubMed] [Google Scholar]
- 10.Riesbeck K, Sigvardson M, Leanderson T, et al. Superinduction of cytokine gene transcription by ciprofloxacine. J Immunol. 1994;153:343–51. [PubMed] [Google Scholar]
- 11.Kletter Y, Singer A, Nagle A, et al. Ciprofloxacine enhances hematopoesis and peritoneal neutrophil function in lethally irradiated bone marrow-transplanted mice. Exp Hematol. 1994;22:360–65. [PubMed] [Google Scholar]
- 12.Blank M, Geaorge J, Fishman P, et al. Ciprofloxacine immunomiodulation of experimental antiphospholipid syndrome associated with elevation of interleukin-3 and granulocyte-macrophage colony-stimulating factor expression. Arthritis Rheum. 1998;41:224–32. doi: 10.1002/1529-0131(199802)41:2<224::AID-ART6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Valera M, Sampedro A, Moreno E, et al. Modification of immune response in mice by ciprofloxacin. Antimicro Agent Chemother. 1995;39:150–54. doi: 10.1128/aac.39.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riesbeck K, Forsgren A. Selective enhancement of synthesis of IL-2 in lymphocytes in the presence of ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1990;9:409–17. doi: 10.1007/BF01979471. [DOI] [PubMed] [Google Scholar]
- 15.Sigsen BH, Swanson VL, Bernstein BH, et al. A histologic evaluation of mixed connective tissue disease in childhood. Am J Med. 1980;68:710. doi: 10.1016/0002-9343(80)90258-2. [DOI] [PubMed] [Google Scholar]
- 16.Peller JS, Gabor GT, Porter JM, Bennett RM, et al. Angiographic findings in mixed connective tissue disease. Correlation with fingernail capillary photomicroscopy and digital pletismography findings. Arthritis Rheum. 1985;28:768. doi: 10.1002/art.1780280707. [DOI] [PubMed] [Google Scholar]
- 17.Jackson J, McDonald M, Casey E, et al. Mixed connective tissue disease with arterial thrombosis, antiphospholi[pid antibodies and heparin induced thrombocytopenia. J Rheumatol. 1990;17:1523–24. [PubMed] [Google Scholar]
- 18.Hainaut P, Lavenne E, Magy JM, Lebacq EG. Circulating lupus type anticoagulant and pulmonary hypertension associated with mixed connective tissue disease. Clin Rheumatol. 1986;5:96–101. doi: 10.1007/BF02030976. [DOI] [PubMed] [Google Scholar]
- 19.Cosnes J, Robert A, Levy VG, Darnis F. Budd-Chiari syndrome in a patient with mixed connective tissue disease. Dig Dis Sci. 1980;25:467–69. doi: 10.1007/BF01395513. [DOI] [PubMed] [Google Scholar]
- 20.Ueda Y, Yamauchi Y, Makizumi K, et al. Successful treatment of acute right cardiac failure due to pulmonary thromboembolism in mixed connective tissue disease. Jpn J Med. 1991;30:568–72. doi: 10.2169/internalmedicine1962.30.568. [DOI] [PubMed] [Google Scholar]
- 21.Rademaker M, Thomas RHM, Provost C, et al. Prolonged increase in digital blood flow following iloprost infusion in patients with systemic sclerosis. Postgrad Med J. 1987;63:617–20. doi: 10.1136/pgmj.63.742.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wigley FM, Wise RA, Seiboid JR, et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. Ann Intern Med. 1994;120:199–206. doi: 10.7326/0003-4819-120-3-199402010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Della Bella S, Molteni M, Mocellin C, et al. Novel mode of action of iloprost: in vitro down-regulation of endothelial cell adhesion molecules. Prostaglandins Other Lipid Mediat. 2001;65:73–83. doi: 10.1016/s0090-6980(01)00131-9. [DOI] [PubMed] [Google Scholar]
- 24.Balbir-Gurman A, Braun-Moscovici Y, Livshitz V, et al. Antioxidant status after iloprost treatment in patients with Raynaud’s phenomenon secondary to systemic sclerosis. Clin Rheumatol. 2007;26(9):1517–21. doi: 10.1007/s10067-007-0613-2. [DOI] [PubMed] [Google Scholar]
- 25.Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol. 2001;2:219–27. doi: 10.2165/00128071-200102040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Sherman RA, Sherman J, Gilead l, et al. Maggot debridgement therapy in outpatients. Arch Phys Med Rehabil. 2001;82:1226–29. doi: 10.1053/apmr.2001.24300. [DOI] [PubMed] [Google Scholar]
- 27.Shoenfeld Y, Krause I. IVIG for autoimmune, fibrosis and malignant conditions: our experience with 200 patients. J Clin Immunol. 2004;24:107–14. doi: 10.1023/b:joci.0000019809.55787.ec. [DOI] [PubMed] [Google Scholar]
- 28.Dirschka T, kastner U, Behrens S, Altmeyer P. Successful treatment of pyoderma gangrenosum with intravenous human immunoglobulin. J Am Acad Dermatol. 1998;39:789–90. doi: 10.1016/s0190-9622(98)70052-0. [DOI] [PubMed] [Google Scholar]
- 29.Zwaan SE, Iland HJ, Damian DL. Treatment of refractory pyoderma gangrenosum with intravenous immunoglobulin. Austral J Dermatol. 2009;50:56–59. doi: 10.1111/j.1440-0960.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 30.Korber A, Lehnen M, Rietkotter J, et al. Successful therapy of a rheumatoid leg ulcer with intravenous immunoglobulins. Hautarzt. 2006;57:1106–10. doi: 10.1007/s00105-005-1073-8. [in German] [DOI] [PubMed] [Google Scholar]
- 31.Ravat FE, Evans AV, Russell-Jones R. Response of livedoid vasculitis to intravenous immunoglobulin. Br J Dermatol. 2002;147:166–69. doi: 10.1046/j.1365-2133.2002.04755.x. [DOI] [PubMed] [Google Scholar]
- 32.Balbir-Gurman A, Nahir AM, Braun-Moscovici Y. Intravenous immunoglobulins in polyarthritis nodosa restricted to the limbs: case reports and review of the literature. Clinc Exp Rheum. 2007;25(1 Suppl 44):S28–30. [PubMed] [Google Scholar]
- 33.Maillard H, Szczesniak S, Martin L, et al. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;126:125–29. [in French] [PubMed] [Google Scholar]
- 34.Ong CS, Benson EM. Successful treatment of chronic leucocytoclastic vasculitis and persistent ulceration with intravenous immunoglobulin. Br J Dermatol. 2000;143:445–73. doi: 10.1046/j.1365-2133.2000.03681.x. [DOI] [PubMed] [Google Scholar]
- 35.Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513–18. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- 36.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–70. [PubMed] [Google Scholar]
- 37.Kime R, Im J, Moser D, et al. Noninvasive determination of exercise-induced vasodilatation during bicycle exercise using near infrared spectroscopy. Med Sci Monit. 2009;15(3):CR89–94. [PubMed] [Google Scholar]
- 38.Melamed Y, Bitterman H. Non-healing wounds and hyperbaric oxygen: a growing awareness. Editorial. IMAJ. 2009;11:78–80. [PubMed] [Google Scholar]