Abstract
Thrombophlebitis of the jugular vein is commonly observed in horses, particularly during intensive care, and leads to local and systemic inflammatory responses as well as head and neck circulatory impairment. Thrombolytic therapy is widely used in human practice with the aim of thrombus dissolution and recanalization of the injured vessels. There are similarities between human and horse coagulation and fibrinolytic processes. This review examines the fibrinolytic system, thrombus formation, and the clinical management of jugular thrombophlebitis, including thrombolytic therapy. There is evidence that early regional thrombolytic therapy for jugular thrombophlebitis in horses may be effective to achieve sustained recanalization.
Résumé
Thrombophlébite jugulaire chez les chevaux : revue de la fibrinolyse, de la formation de thrombus et de la gestion clinique. La thrombophlébite de la veine jugulaire est communément observée chez les chevaux, particulièrement durant les soins intensifs, et cause des réactions inflammatoires locales et systémiques ainsi que des altérations de la circulation dans la tête et le cou. La thérapie thrombolytique est couramment utilisée en pratique humaine dans le but de dissoudre les thrombus et de recanaliser les vaisseaux blessés. Il y a similitudes entre les processus coagulant et fibrinolytique des humains et des chevaux. Cette revue examine le système fibrinolytique, la formation de thrombus et la gestion clinique de la thrombophlébite jugulaire, incluant la thérapie thrombolytique. Il y a des preuves que la thérapie thrombolytique régionale précoce pour la thrombophlébite jugulaire chez les chevaux peut être efficace pour la réalisation d’une recanalisation durable.
(Traduit par Isabelle Vallières)
Introduction
Thrombophlebitis of the jugular vein in horses is a commonly observed, undesirable iatrogenic complication, particularly during intensive care. Several drugs are widely used for acute thrombolytic therapy in human practice but there are few reports on the use of thrombolytic agents to treat thrombosis in horses (1–4). This review examines the fibrinolytic system, thrombus formation, and the clinical management of jugular thrombophlebitis, including thrombolytic therapy in horses. The review also highlights the evidence supporting the use of streptokinase (SK) as a regional treatment for clinical thrombosis in horses.
The fibrinolytic system
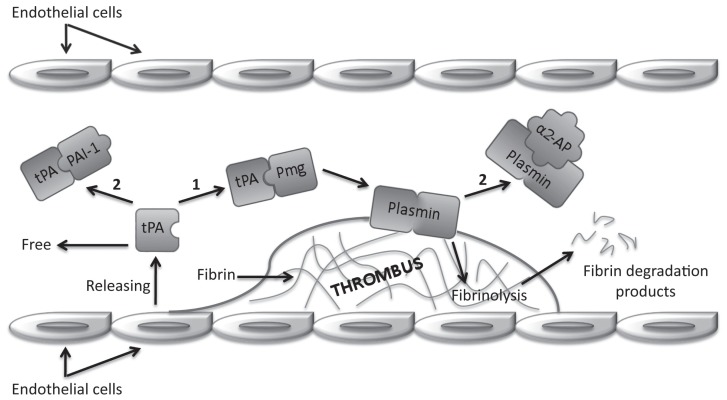
In 1958, Astrup (5) proposed the concept of dynamic equilibrium for coagulation and fibrinolytic systems. In this delicate balance, the fibrinolytic process breaks down fibrin which is continuously deposited throughout the cardiovascular system as a result of limited physiological activation of the coagulation. The coagulation and fibrinolytic systems interact through feedback mechanisms that are not fully understood (6). Fibrinolysis is regulated such that unwanted fibrin thrombi are removed from circulation, whereas fibrin in wounds persists to prevent blood loss and maintain hemostasis (7–9). The vascular endothelium, a highly specialized multifunctional organ, is intimately involved with regulation of hemostasis, supporting anticoagulant mechanisms, including fibrinolysis and inhibition of platelets and serine proteases, to maintain blood fluidity (10). The cascade of reactions leading to thrombus dissolution is schematically illustrated in Figure 1.
Figure 1.
Schematic representation of fibrinolysis showing tissue plasminogen activator (tPA) pathway. Tags: plasminogen (Pmg); plasminogen activator inhibitor-1 (PAI-1); alpha2-antiplasmin (α2-AP); activation (1); inhibition (2).
The key enzyme in the fibrinolytic system is the glycoprotein plasminogen which is produced in the liver (6). The inactive proenzyme plasminogen is converted to the active enzyme plasmin by 2 specific serine proteases: the tissue-type plasminogen activator (tPA), released from endothelial cells in response to stasis or vessel wall injury, and the urokinase type plasminogen activator (uPA), also called urokinase. The tPA-mediated pathway is primarily involved in fibrin homeostasis, and the uPA-mediated pathway is involved in cell migration and tissue remodeling (11). Activation of plasminogen to plasmin occurs by the cleavage of an arginine-valine bond, which produces changes in conformation, leading to an increased affinity for both degradation substrates and activators (6). Plasmin, a relatively nonspecific protease, has the capacity to hydrolyze fibrin and various plasma proteins, including fibrinogen and coagulation factors (6,7,12). Plasmin also activates matrix metal-loproteinases that, in turn, degrade the extracellular matrix (11).
Fibrinolytic activity is modulated by plasminogen activators and inhibitors (8,10,12). The plasminogen activator inhibitor 1 (PAI-1) is a fast-acting inhibitor of tPA and uPA; PAI-2 is secreted in small amounts under normal conditions, but may be released in high concentration from damaged endothelium (10). The most important inhibitors that act at the plasmin level are alpha2-antiplasmin and alpha2-macroglobulin (8,12). Alpha2-antiplasmin, a single-chain glycoprotein protease inhibitor, is the main physiologic inhibitor of plasmin (6). Plasma concentrations of alpha2-antiplasmin are sufficient to inhibit about 50% of potential plasmin (7). The half-life of alpha2-antiplasmin and plasmin complex is approximately 12 h (6). When massive activation of plasminogen occurs, such as during thrombolytic therapy, alpha2-antiplasmin is depleted, and the high free plasmin levels lead to a “systemic lytic state,” increasing the risk of bleeding from wounds (7). The thrombin activatable fibrinolysis inhibitor (TAFI) is a plasma protein that attenuates the conversion of plasminogen to plasmin. During inflammatory processes, the anti-coagulant effects of protein C are impaired and activation of TAFI is retained. Therefore, fibrinolysis is decreased, adding to the prothrombotic coagulopathy associated with sepsis (9).
Tests for the assessment of fibrinolysis in horses include tests for fibrinogen concentration, fibrin degradation products (FDPs), D-dimer (DD) levels, and thromboelastography (TEG). Fibrinogen concentrations may be difficult to interpret in critically ill horses as the levels change with both inflammation and coagulopathies, although low levels may support increased fibrinolysis. Both FDPs and DD result from fibrinolysis, but FDPs result from the degradation of both fibrin and fibrinogen (13), while DDs (formed only by the plasmin digestion of cross-linked fibrin) are considered specific for fibrin (13,14). Fibrin monomers (FM), the initial products of fibrin formation, form high molecular weight complexes with fibrinogen and FDPs and is an accurate indicator of an activated state of coagulation. Fibrin monomers may be deposited locally, in cases of thrombosis, or diffusely, in cases of disseminated intra-vascular coagulation (DIC) in humans (15,16). In horses, FM also indicates the coagulation state. Clinical studies evaluating horses after colic surgery reported high levels of FM before the occurrence of jugular vein thrombosis (17) and DIC (18). Antithrombin (AT) inactivates thrombin and some coagulation factors, and is considered the main inhibitor of coagulation (19). In horses undergoing colic surgery, reductions in AT corresponded to the severity of intestinal lesions (20). Evaluating coagulation and fibrinolysis indicators, Feige et al (20) showed that activation of coagulation and fibrinolysis increased after the colic surgical procedure, marked by a prolonged activated partial thromboplastin time, and by increased concentrations of DD and FM. The authors also reported that surgical trauma per se did not influence coagulation, and changes were due to the primary disease. The study concluded that high FM and DD concentrations represent an increased risk for the development of a coagulopathy after colic surgery.
Thromboelastography measures global hemostatic function of whole blood in vitro, documenting the interaction between platelets and coagulation cascade proteins (21) and provides information on clot kinetics and mechanical properties from formation through maturation and fibrinolysis. The use of whole blood allows the analysis of cellular and plasma components, providing a global assessment of hemostatic potential (22,23). Validation of TEG in horses and establishment of the first reference values were performed by Paltrinieri et al (24). Thromboelastography may be a clinically useful tool in horses. For example, in horses presenting with gastrointestinal diseases, TEG was an effective method to determine coagulation and fibrinolysis changes, as well as to distinguish hypocoagulation and hypercoagulation states (22,25).
Thrombus formation
The equilibrium proposed by Astrup (5) is easily overwhelmed during pathologic coagulation states. In such states, the fibrinolytic system fails to maintain fluidity of the blood in the affected vessels, resulting in thrombosis (6). Equine diseases commonly associated with coagulation abnormalities include gastrointestinal diseases and sepsis, often associated with endotoxemia or the systemic inflammatory response syndrome (SIRS) (13,26). Other conditions in horses associated with coagulopathies include genetic coagulation defects, neoplasia, protein-losing enteropathy, and protein-losing nephropathy (26).
Horses with gastrointestinal disorders and sepsis may develop coagulopathies characterized by excessive activation of coagulation, and impairment of the fibrinolysis and anti-coagulant pathways (9,13,27,28). The degree of abnormal coagulation with gastrointestinal diseases may be mild to marked, depending upon the severity of the disorder. Simple obstructions and displacements usually produce a mild to moderate activation of the coagulation system, usually compensated by the fibrinolytic system. More severe problems such as intestinal strangulation or enteritis may produce a marked prothrombotic state, which can lead to disseminated intravascular coagulation (9,28). Medical treatment of acute abdomen, or postoperative care of horses undergoing surgery for gastrointestinal lesions is often intensive and frequently necessitates long-term maintenance of a jugular catheter for administration of intravenous fluids and medications. If these horses have concurrent coagulopathies, in association with jugular vein trauma and inflammation occurring with catheterization, they are at increased risk for the development of thrombophlebitis of the jugular vein (13,28). Mair and Smith (29) reported that 8.3% of 252 horses that underwent surgical treatment of colic developed jugular vein thrombophlebitis.
The imbalance leading to thrombus formation may be a result of multiple factors, including vessel injury or other intrinsic vessel wall lesion, a low-flow state or venous stasis, and blood hypercoagulability (6,30). This classic triad, describing the pathogenesis of thrombosis, was first outlined by Virchow in 1856 (cited in reference 30). Experimentally and clinically, it is now recognized that at least 2 of these 3 postulated factors in combination are essential in the progress of venous thrombosis. In humans, decreased blood flow or stasis seems to be the dominant component (31). However in horses, local trauma is the most important factor of this triad in the development of jugular vein thrombosis (32).
Thrombosis begins with platelets adhering to macromolecules in the subendothelial regions of the injured blood vessel to form the primary hemostatic plug. The platelets then stimulate local activation of plasma coagulation factors, leading to the generation of a fibrin clot that reinforces the platelet aggregate. Within an intact blood vessel, thrombosis is prevented by the body’s anticoagulant mechanisms. Prostacyclin (PGI2), anti-thrombin, heparan sulfate proteoglycans, protein C, protein S, ADPase, and TF pathway inhibitor (TFPI) are examples of natural thrombosis inhibitors (7,9,33,34). Activated protein C and protein S, in the circulation or on the endothelium surface, block the inhibitory effect of PAI-1. Therefore, plasminogen may exert its function leading to fibrinolysis, thereby preventing thrombotic processes (10).
Jugular thrombophlebitis in horses
Thrombophlebitis is defined as venous thrombosis secondary to inflammation of the vessel wall (35–37). Infection can complicate and enhance this venous disorder. In septic thrombophlebitis, there is bacterial or, rarely, fungal colonization of the vessel wall, thrombus, and, occasionally, of the surrounding perivascular tissues (38). Blood flow is impaired due to reduced vascular lumen by partial or complete occlusion (38,39). Thrombophlebitis most commonly affects the jugular veins in horses because they are the most commonly used site to access venous circulation. The disease is associated with poor venipuncture technique, prolonged or improper placement of indwelling catheters, and injections of irritating drugs that lead to mechanical or chemical injury of the vessel wall (30,36,40–44). An atypical etiology of jugular thrombophlebitis, extending directly from a penetrating buccal ulcer in a horse, was described by Matsuda et al (45).
The development of thrombophlebitis following catheterization is dependent on catheter composition, venipuncture technique, cannula size, catheter maintenance, pH of the infusate solution, duration of infusion, and presence of bacteria (46). All of these factors underscore the importance of using aseptic techniques to implant and maintain venous catheters.
A bacteriological study assessing techniques for disinfecting sites prior to the insertion of jugular intravenous catheters in horses reported that chlorhexidine and povidone-iodine were equally effective when used on skin after the hair had been clipped (47). The authors concluded that clipping or shaving the hair over the veins prior to disinfection is recommended, but there were no differences when the hair was left long, clipped, or shaved. Therefore, the skin can be disinfected effectively with either agent without clipping or shaving the hair when rapid intravenous catheterization is a clinical priority.
The pharmaceuticals most frequently associated with the development of thrombophlebitis include glycerol guaiacolate, thiopental, calcium gluconate, phenylbutazone, and oxytetracycline (38,39). Phenylbutazone, widely used for anti-inflammatory therapy of musculoskeletal system injuries of athletic horses, is commonly associated with thrombophlebitis when it is accidently infused in the perivascular region (39,48).
Clinical signs associated with aseptic jugular vein thrombosis are generally limited to localized firmness and a cord-like jugular vein. Venous distention proximal to the thrombus site is observed when there is complete occlusion. When bacteria are seeded in thrombi resulting in septic thrombophlebitis, horses have intermittent fever, depression, and neck stiffness. Palpation elicits local swelling, heat, pain, and a cord-like jugular vein. Generally, leukocytosis, neutrophilia, and hyperfibrinogenemia are present (49,50). As reported by Ryu et al (51), septic thrombi in the jugular vein may also lead to pleuropneumonia via the pulmonary circulation.
The diagnosis of septic or nonseptic thrombophlebitis is primarily based on history and physical signs (38,39,49). The disease is evaluated with hematology tests, ultrasonography, contrast angiograms, and bacteriologic confirmation (35,49,50,52,53). Ultrasonography of the vein and surrounding tissue can be used to specifically characterize the thrombus and monitor the extent and progress of the lesion (37,54). The jugular vein and its valves can be readily identified (35). In cases of thrombophlebitis, ultrasonography allows an evaluation of vessel walls, blood flow compromise, and presence, extension and shape of the thrombus, as well as compensatory vascularization and recanalization characteristics (55). The sonographic appearance of the normal jugular vein is that of an anechoic circular or tubular structure, with identification of a more hyperechoic vessel wall (38). Inflamed jugular veins often appear thick-walled, and the thrombus is imaged as a partially or totally occlusive hyperechoic structure adhering to the vessel wall. In cases of partial obstruction, the blood flow appears as an anechoic area between the thrombus and opposite vessel wall where the thrombus is connected (38,56,57). If only partial obstruction to blood flow is present, distension of the vein by distal digital occlusion helps to prevent collapse during ultrasonographic evaluation (55). A septic thrombus appears as a hyperechoic heterogeneous cavitating mass in which the anechoic areas represent fluid accumulation secondary to infection and necrosis (38,50,54). Anaerobic bacterial infections often show numerous hyperechoic areas creating an acoustic shadow due to gas production (38). Blood flow across the affected vessel may be evaluated by venography, or with pulsed-wave or color-coded Doppler (50).
If a catheter is still in place when a thrombosis is discovered, the catheter should be carefully withdrawn and the tip cultured (26,35). Identification of the infectious agent and its drug susceptibility is important for treatment (35). While the catheter remains in situ, the thrombus will not dissolve, and it may grow (58). Furthermore, the injured vessel should be preserved, and venipuncture should be avoided. The opposite jugular vein should also be preserved to avoid the risk of bilateral thrombophlebitis. Lateral thoracic, cephalic, and saphenous veins are other options for drug administration and venous blood collection (59). In a retrospective study, Dolente et al (26) reported severe intestinal disease, hypoproteinemia, salmonellosis, and endotoxemia as potential risk factors for development of catheter-associated jugular thrombophlebitis in horses.
Both medical (35,43,60,61) and surgical (40,42,62–66) treatments for thrombophlebitis are reported. Local treatment consists of hot packs and hydrotherapy applied over the swollen vein 3 or 4 times daily and the application of dimethyl sulfoxide (DMSO) solution on the affected area. Non-steroidal anti-inflammatory drugs may be useful in reducing pain and inflammation. Systemic antibiotics are indicated for sepsis (35,43,50,61). Bonagura et al (50) suggested metronidazole at 15 mg/kg body weight (BW), PO, q6h, or at 25 mg/kg BW, PO, q12h in cases of anaerobic infection. If the thrombotic lesion is associated with a generalized coagulopathy, heparin therapy [unfractionated, 40 to 100 IU/kg BW, IV or SQ, q6h; low molecular weight (Dalteparin), 50 to 100 IU/kg BW, SQ, q24h] may be beneficial (67). Efficacy and side effects of low molecular weight heparin (LMWH) compared to unfractionated heparin (UH) have been evaluated in horses with gastrointestinal diseases. At a dose of 50 IU/kg BW, q24h, LMWH decreases the incidence of jugular thrombosis compared to treatment with UH in horses having colic surgery (68). Aspirin at a dose of 20 mL/kg BW every other day is also recommended for the treatment of thrombophlebitis to inhibit platelet activation and aggregation (30). Scott et al (60) reported 2 cases of jugular vein thrombophlebitis successfully treated by warfarin anticoagulation. However, the authors recommend this therapy only in situations in which the coagulation status can be monitored by daily laboratory determinations of prothrombin time.
When jugular vein thrombophlebitis is refractive to medical treatment, surgical techniques including phlebotomy, thrombectomy with Fogarty’s catheter, or venous grafts may be performed to restore blood flow (40,42,62–66). Dias et al (57) performed an ultrasonographic evaluation during the acute phase of experimental jugular vein thrombophlebitis in horses and demonstrated that the major thrombus development occurs in the early stages of injury. These findings suggest that the first 24 h may represent the most effective intervention period. In horses, the complications associated with jugular vein thrombophlebitis include pleuropneumonia, bacterial endocarditis, pulmonary thromboembolism, septicemia, and other conditions associated with metastasis of septic thrombi (44).
The long-term outcome for a thrombus is variable. Recanalization of the vein may take place. The thrombus may undergo fibrous organization without recanalization or collateral circulation may develop (44). Hussni et al (69) observed via angiography that collateral vascularization develops within 1 y in horses submitted to a unilateral jugular vein resection that restores the venous drainage of the head and neck. If the jugular venous obstruction persists and collateral vascularization does not occur, especially when both jugular veins are involved, dysphagia and dyspnea due to impaired venous return from the head is likely to limit athletic performance (38,42,50). Moreau and Lavoie (44) suggested that thrombophlebitis did not affect the athletic performance of horses used for pleasure riding and other nonracing activities, even when the horses suffered bilateral occlusion. However, thrombophlebitis in racing Standardbreds was associated with a decreased chance of return to racing.
Thrombolytic therapy and streptokinase
The medical treatments for equine jugular vein thrombophlebitis are often ineffective, and restoration of blood flow is typically only achieved with complex and expensive surgical procedures. In human medicine, thrombolytic therapy involving fibrinolytic agents has revolutionized the treatment of diverse circulatory disorders, such as pulmonary embolism, deep-vein thrombosis, and myocardial infarction (12). It has been possible to treat intravascular thrombosis and achieve complete dissolution by using drugs capable of stimulating the fibrinolytic system (6). Thrombolytic therapy consists of the pharmacological dissolution of the blood clot by intravenous infusion of plasminogen activators that activate the intrinsic fibrinolytic system components (11,12). Thrombolytic drugs dissolve both pathological thrombi and fibrin deposits at sites of vascular injury, with the risk of hemorrhage as the major side effect (7). Streptokinase (SK) is a nonenzymatic protein produced by strains of β-hemolytic streptococci (6,11,12). The capacity of SK to promote the lysis of blood clots was first described by Tillett in 1933 (cited in reference 70); the effect is thought to be mediated by enzymatic action on fibrin (70). Streptokinase combines with plasminogen to form a stable activator complex that has enzymatic activity (71). This SK-activator complex then activates the fibrinolytic mechanism by converting uncomplexed plasminogen to plasmin. The SK-plasminogen complex is slowly converted to a SK-plasmin form, which can also activate and convert plasminogen. The initial activity is mediated by the SK-plasminogen complex, whereas the later activity is mediated by the SK-plasmin form (6). Streptokinase is a foreign protein, and its introduction into the circulatory system can elicit an anaphylactic response (8). Plasma antibodies against SK are normally present as a result of prior streptococcal infections. Therefore, systemic lytic therapy only becomes effective when enough of the drug has been administered to overcome these antibodies (7). Lytic agents can be administered systemically or regionally (6).
In the attempt to avoid the hemorrhagic consequences of thrombolytic therapy, Dotter et al (72) proposed a low dose, local infusion of SK for the treatment of arterial occlusive disorders. Due to the smaller total dose of SK in local application, there were fewer complications than with systemic therapy. Regional thrombolytic therapy was further developed, and local targeted instillation through a catheter implanted into thrombotic material was proposed (6,73). The technique makes local fibrinolysis possible, and, importantly, this process is partially protected from circulating inhibitors. Regional thrombolytic therapy with SK has been used successfully in human practice for venous thrombotic disorders of superficial veins, such as the axillosubclavian vein (6). The use of SK may be successful for jugular vein thrombophlebitis in horses, given the similarity between horse and human thrombosis development processes (74), and the superficial localization and easy access to the jugular vein in horses. Nevertheless, there are few investigations analyzing SK fibrinolytic activity in horses. The use of SK in horses was first reported in 1966 and 1968 to treat aortic and left iliac thrombosis, respectively. Streptokinase was administrated in association with streptodornase, plasminogen, and sodium gluconate. Unfortunately, the authors could not specify which fibrinolytic agent led to case resolution (1,2). Lions et al (3) described the administration of SK to treat jugular thrombophlebitis in horses, showing evidence of SK fibrinolytic activity; however, their methods are not fully established. According to Dias et al (4), regional thrombolytic therapy using a bolus administration of 100 mL of SK solution (7500 IU of SK per mL of 0.9% sodium chloride solution) may be effective for resolving induced jugular vein thrombosis in horses, and providing transitory recanalization.
Other applications for fibrinolytic therapy have been reported besides jugular thrombophlebitis in horses. Clark-Price et al (75) utilized recombinant tPA in the anterior chamber of each eye of a foal with fibrin accumulation associated with endotoxemia and septicemia, providing fibrin clot clearance and restoring normal vision. Hilton and Pusterla (76) also used fibrinolytic therapy in the management of septic pleuropneumonia in a horse.
Early regional thrombolytic therapy for jugular thrombophlebitis in horses may be effective to achieve sustained recanalization. A thorough understanding of the fibrinolytic system and the potential complications associated with its modification is an essential foundation for advances in thrombolytic therapy. Additional research is needed to expand our knowledge of the fibrinolytic system, and to develop new clinical applications to address coagulation problems in horses. The authors hope to stimulate new investigations analyzing drugs capable of activating the fibrinolytic system in horses, and to promote future development of novel treatments for jugular thrombophlebitis and other diseases associated with fibrin accumulation in horses.
Acknowledgments
We acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) for financial assistance. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Tillotson PJ, Kopper PH. Treatment of aortic thrombosis in a horse. J Am Vet Med Assoc. 1966;149:766–767. [PubMed] [Google Scholar]
- 2.Branscomb BL. Treatment of arterial thrombosis in a horse with sodium gluconate. J Am Vet Med Assoc. 1968;152:1643–1644. [PubMed] [Google Scholar]
- 3.Lions JA, Carette O, Broucker CA, Tainturier B, Ribot X. Utilisation de la streptokinase dans le traitement des thrombophlébites de la jugulaire chez le cheval : à propos de 2 cas cliniques. Bull Soc Vét Prat de France. 2008;92:17–22. [Google Scholar]
- 4.Dias DPM, Teixeira LG, Canola PA, et al. Streptokinase as a treatment for jugular vein thrombophlebitis in horses. Proc 12th World Equine Veterinary Association (WEVA) Congress; Hyderabad, India. 2011. [Google Scholar]
- 5.Astrup T. The haemostatic balance. Thromb Diath Haemost. 1958;2:347–357. [PubMed] [Google Scholar]
- 6.Kashyap VS, Quinones-Baldrich WJ. Principles of thrombolytic therapy. In: Kashyap VS, Quinones-Baldrich WJ, editors. Vascular Surgery. 5th ed. Philadelphia, Pennsylvania: Saunders; 2000. pp. 457–475. [Google Scholar]
- 7.Majerus PW, Broze GJ, Jr, Miletich JP, Tollefsen DM. Anticoagulant, thrombolytic, and antiplatelet drugs. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. 4th ed. New York, New York: Pergamon Press; 1990. pp. 1311–1331. [Google Scholar]
- 8.Banerjee A, Chisti Y, Banerjee UC. Streptokinase — a clinically useful thrombolytic agent. Biotechnol Adv. 2004;22:287–307. doi: 10.1016/j.biotechadv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Hopper K, Bateman S. An updated viewof hemostasis: Mechanisms of hemostatic dysfuntion associated with sepsis. J Vet Emerg Crit Care. 2005;15:83–91. [Google Scholar]
- 10.Duffy TC, Kirby R, Rudloff E. Critical role of the vascular endothelial cell in health and disease: A review article. J Vet Emerg Crit Care. 2004;14:84–99. [Google Scholar]
- 11.Collen D. The plasminogen (fibrinolytic) system. J Thromb Haemost. 1999;82:259–270. [PubMed] [Google Scholar]
- 12.Kunamneni A, Abdelghani TTA, Ellaiah P. Streptokinase — the drug of choice for thrombolytic therapy. J Thromb Thrombolysis. 2007;23:9–23. doi: 10.1007/s11239-006-9011-x. [DOI] [PubMed] [Google Scholar]
- 13.Schaer BLD, Epstein K. Coagulopathy of the critically ill equine patient. J Vet Emerg Crit Care. 2009;19:53–65. doi: 10.1111/j.1476-4431.2009.00390.x. [DOI] [PubMed] [Google Scholar]
- 14.Dempfle CA. Use of D-dimer assays in the diagnosis of venous thrombosis. Seminar Thromb Haemost. 2000;26:631–641. doi: 10.1055/s-2000-13221. [DOI] [PubMed] [Google Scholar]
- 15.Haselager EM, Vreeken J. Clinical significance of “circulating fibrin monomers”. J Clin Pathol. 1981;34:468–472. doi: 10.1136/jcp.34.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempfle CA. The use of soluble fibrin in evaluating the acute and chronic hypercoagulable state. Thromb Haemost. 1999;82:673–683. [PubMed] [Google Scholar]
- 17.Feige K, Dempfle CE, Balestra E. Measurement of soluble fibrin in horses using Enzymun-Test FM. Thromb Haemost. 1999;(Suppl):2472. [Google Scholar]
- 18.Stern-Balestra E. Doctoral thesis. University of Zurich; Zurich, Switzerland: 2000. Bestimmung von löslichen fibrinkomplexen beim pferd als möglichkeit der diagnostik einer aktivierten blutgerinnung. [Google Scholar]
- 19.Holland M, Kelly AB, Snyder JR, Steffey EP, Willits N, McNeal D. Antithrombin activity in horses with large colon torsion. Am J Vet Res. 1986;47:897–900. [PubMed] [Google Scholar]
- 20.Feige K, Kästner SBR, Dempfle CE, Balestra E. Changes in coagulation and markers of fibrinolysis in horses undergoing colic surgery. J Vet Med A Physiol Pathol Clin Med. 2003;50:30–36. doi: 10.1046/j.1439-0442.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- 21.Souza MHL, Elias DO. Tromboelastografia na circulação extracorpórea. Boletim Informativo Tecnologia Extracorpórea. 2008;6:1–4. [Google Scholar]
- 22.Epstein KL, Brainard BM, Gomez-Ibanez SE, Lopes MAF, Barton MH, Moore JN. Thromboelastography in horses with acute gastrointestinal disease. J Vet Inter Med. 2011;25:307–314. doi: 10.1111/j.1939-1676.2010.0673.x. [DOI] [PubMed] [Google Scholar]
- 23.Kol A. The interpretation of thromboelastography tracings: Many (more) rivers to cross. Vet J. 2012;191:275–276. doi: 10.1016/j.tvjl.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Paltrinieri S, Meazza C, Giordano A, Tunesi C. Validation of thrombo-elastometry in horses. Vet Clin Pathol. 2008;37:277–285. doi: 10.1111/j.1939-165X.2008.00052.x. [DOI] [PubMed] [Google Scholar]
- 25.Mendez-Angulo JL, Mudge MC, Vilar-Saavedra P, Stingle N, Couto G. Thromboelastography in healthy horses and horses with inflammatory gastrointestinal disorders and suspected coagulopathies. J Vet Emerg Crit Care. 2010;20:488–493. doi: 10.1111/j.1476-4431.2010.00576.x. [DOI] [PubMed] [Google Scholar]
- 26.Dolente BA, Beech J, Lindborg S, Smith G. Evaluation of risk factors for development of catheter-associated jugular thrombophlebitis in horses: 50 cases (1993–1998) J Am Vet Med Assoc. 2005;227:1134–1141. doi: 10.2460/javma.2005.227.1134. [DOI] [PubMed] [Google Scholar]
- 27.Monreal L, Anglés A, Espada Y, Monasterio J, Monreal M. Hyperco-agulation and hypofibrinolysis in horses with colic and DIC. Equine Vet J. 2000;32:19–25. doi: 10.1111/j.2042-3306.2000.tb05329.x. [DOI] [PubMed] [Google Scholar]
- 28.Monreal L, Cesarini C. Coagulopathies in horses with colic. Vet Clin North Am Equine Pract. 2009;25:247–258. doi: 10.1016/j.cveq.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Mair TS, Smith LJ. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 2: Short-term complications. Equine Vet J. 2005;37:303–309. doi: 10.2746/0425164054529364. [DOI] [PubMed] [Google Scholar]
- 30.Sellon DC, Wise LN. Disorders of the hematopoietic system. In: Reed SM, Bayly WM, Sellon DC, editors. Equine Internal Medicine. 3rd ed. St. Louis, Missouri: Saunders; 2010. pp. 730–776. [Google Scholar]
- 31.Mammen EF. Pathogenesis of venous thrombosis. Chest Suppl. 1992;102:640S–644S. doi: 10.1378/chest.102.6_supplement.640s. [DOI] [PubMed] [Google Scholar]
- 32.Lankveld DPK, Ensink JM, Van Dijk P, Klein WR. Factors influencing the occurrence of thrombophlebitis after post-surgical long-term intravenous catheterization of colic horses: A study of 38 cases. J Vet Med A Physiol Pathol Clin Med. 2001;48:545–552. doi: 10.1046/j.1439-0442.2001.00383.x. [DOI] [PubMed] [Google Scholar]
- 33.Caprino R, Togna AR, Stella C, Togna G. An in vitro method for evaluating vascular endothelial ADPase activity. J Pharmacol Toxicol Methods. 1996;35:153–157. doi: 10.1016/1056-8719(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 34.Esmon CT. Role of coagulation inhibitors in inflammation. Thromb Haemost. 2001;86:51–56. [PubMed] [Google Scholar]
- 35.White N., II . Intensive care, monitoring and complications of acute abdominal disease. In: White N II, editor. The Equine Acute Abdomen. Philadelphia, Pennsylvania: Lea & Febiger; 1990. pp. 309–335. [Google Scholar]
- 36.Ettlinger JJ, Palmer JE, Benson C. Bacteria found on intravenous catheters removed from horses. Vet Rec. 1992;130:248–249. doi: 10.1136/vr.130.12.248. [DOI] [PubMed] [Google Scholar]
- 37.Klohnen A. New perspectives in postoperative complications after abdominal surgery. Vet Clin North Am Equine Pract. 2009;25:341–350. doi: 10.1016/j.cveq.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Edens LM. Iatrogenic thrombophlebitis. In: Colahan PT, Mathew IG, Merritt AM, Moore JN, editors. Equine Medicine and Surgery. 5th ed. Vol. 1. St. Louis, Missouri: Mosby; 1999. pp. 416–419. [Google Scholar]
- 39.Thomassian A. Afecções sanguíneas e vasculares. In: Thomassian A, editor. Enfermidades dos Cavalos. 3rd ed. São Paulo, Brazil: Varela; 1996. pp. 519–532. [Google Scholar]
- 40.Cannon JH, Rantanen NW, Grant BD, Keck MT. Jugular venous prothesis on the horse: A preliminary study. J Equine Vet Sci. 1983;3:185–189. [Google Scholar]
- 41.Knottenbelt DC, Pascoe RR. Disorders of the cardiovascular system. In: Knottenbelt DC, Pascoe RR, editors. A Color Atlas of Diseases and Disorders of the Horse. Barcelona, Spain: Wolfe; 1994. pp. 157–188. [Google Scholar]
- 42.Rijkenhuizen ABM, Van Swieten HA. Reconstruction of the jugular vein in horses with post thrombophlebitis stenosis using saphenous vein graft. Equine Vet J. 1998;30:236–239. doi: 10.1111/j.2042-3306.1998.tb04493.x. [DOI] [PubMed] [Google Scholar]
- 43.Divers TJ. Prevention and treatment of thrombosis, phlebitis and laminitis in horses with gastrointestinal diseases. Vet Clin North Am Equine Pract. 2003;19:779–790. doi: 10.1016/j.cveq.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Moreau P, Lavoie JP. Evaluation of athletic performance in horses with jugular vein thrombophlebitis: 91 cases (1988–2005) J Am Vet Med Assoc. 2009;235:1073–1078. doi: 10.2460/javma.235.9.1073. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda K, Suzuki H, Tsunoda N, Taniyama H. Jugular thrombophlebitis developed from buccal ulcer in a thoroughbred horse. J Vet Med Sci. 2010;72:913–915. doi: 10.1292/jvms.09-0571. [DOI] [PubMed] [Google Scholar]
- 46.Dickson LR, Badcoe LM, Burbidge H, Kannegieter NJ. Jugular thrombophlebitis resulting from an anaesthetic induction technique in the horse. Equine Vet J. 1990;22:177–179. doi: 10.1111/j.2042-3306.1990.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 47.Geraghty TE, Love S, Taylor DJ, Heller J, Mellor DJ, Hughes KJ. Assessing techniques for disinfecting sites for inserting intravenous catheters into the jugular veins of horses. Vet Rec. 2009;164:51–55. doi: 10.1136/vr.164.2.51. [DOI] [PubMed] [Google Scholar]
- 48.Beard LA. Princípios de tratamento antimicrobiano. In: Reed SM, Bayly WM, editors. Medicina Interna Eqüina. Rio de Janeiro: Guanabara Koogan; 2000. pp. 135–161. [Google Scholar]
- 49.Dyson SJ. Problems associated with the neck: Neck pain and stiffness, abnormal posture, and forelimb gait abnormalities. In: Robinson NE, editor. Current Therapy in Equine Medicine. 4th ed. Philadelphia, Pennsylvania: Saunders; 1997. pp. 1–6. [Google Scholar]
- 50.Bonagura JD, Reef VB, Schwarzwald CC. Cardiovascular diseases. In: Reed SM, Bayly WM, Sellon DC, editors. Equine Internal Medicine. 3rd ed. St. Louis, Missouri: Saunders; 2010. pp. 372–487. [Google Scholar]
- 51.Ryu S, Kim J, Bak U, Lee C, Lee YL. A hematogenic pleuropneumonia caused by postoperative septic thrombophlebitis in a Thoroughbred gelding. J Vet Sci. 2004;5:75–77. [PubMed] [Google Scholar]
- 52.Reimer JM. Vasculature (jugular vein and lymphatics) In: Reimer JM, editor. Atlas of Equine Ultrasonography. St. Louis, Missouri: Mosby; 1998. pp. 165–169. [Google Scholar]
- 53.Joffe HV, Goldhaber SZ. Upper-extremity deep vein thrombosis. Circulation. 2002;106:1874–1880. doi: 10.1161/01.cir.0000031705.57473.1c. [DOI] [PubMed] [Google Scholar]
- 54.Gardner SY, Reef VB, Spencer PA. Ultrasonographic evaluation of horses with thrombophlebitis of the jugular vein: 46 cases (1985–1988) J Am Vet Med Assoc. 1991;199:370–373. [PubMed] [Google Scholar]
- 55.Barbosa RG, Borghesan AC, Cerqueira NF, et al. Fisiopatologia da trombose e tromboflebite da veia jugular de equinos: revisão. Vet Zootec. 2009;16:26–37. [Google Scholar]
- 56.Reef VB. Cardiovascular ultrasonography. In: Reef VB, editor. Equine Diagnostic Ultrasound. Philadelphia, Pennsylvania: Saunders; 1998. pp. 215–272. [Google Scholar]
- 57.Dias DPM, Canola PA, Teixeira LG, Albernaz RM, Canola JC, Lacerda-Neto JC. Ultrasonographic evaluation of experimental jugular vein thrombophlebitis in horses. Vet Surg. 2010;39:E21. [Google Scholar]
- 58.Geraghty TE, Love S, Taylor DJ, Heller J, Mellor DJ, Hughes KJ. Assessment of subclinical venous catheter-related diseases in horses and associated risk factors. Vet Rec. 2009;164:227–231. doi: 10.1136/vr.164.8.227. [DOI] [PubMed] [Google Scholar]
- 59.Gardner SY, Donawick WJ. Jugular vein thrombophlebitis. In: Robinson NE, editor. Current Therapy in Equine Medicine. Vol. 3. Philadelphia, Pennsylvania: Saunders; 1992. pp. 406–410. [Google Scholar]
- 60.Scott EA, Byars TD, Lamar AM. Warfarin anticoagulation in the horse. J Am Vet Med Assoc. 1980;177:1146–1151. [PubMed] [Google Scholar]
- 61.Dargatz J, Dargatz D. Intravenous catheters and thrombophlebitis. Reports of Equine Veterinary Meetings. 1993;13:379. [Google Scholar]
- 62.Ben-Chehida N, Bellagha A, Bardi K. Traitement d’une thrombophlébite chez un cheval par une prothèse en polyretrafluoroethylene (PTFE) Prat Vét Equine. 1994;26:169–173. [Google Scholar]
- 63.Wiemer P, Gruys E, Hoeck BV. A study of seven different types of grafts for jugular vein transplantation in the horse. Res Vet Sci. 2005;79:211–217. doi: 10.1016/j.rvsc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Dornbusch PT, Hussni CA, Yoshida WB, Sequeira JL, Vulcano LC, Cillo GP. Implante jugular homólogo fixado em glutaraldeído, nos equinos. Rev Port Ciênc Vet. 2007;102:81–86. [Google Scholar]
- 65.Hussni CA, Dornbusch PT, Yoshida WB, et al. Trombectomia com cateter de Fogarty no tratamento da tromboflebite jugular experimental em equinos. Pesqui Vet Bras. 2009;29:45–51. [Google Scholar]
- 66.Russel TM, Kearney C, Pollock PJ. Surgical treatment of septic jugular thrombophlebitis in nine horses. Vet Surg. 2010;39:627–630. doi: 10.1111/j.1532-950X.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- 67.Orsini JA, Divers TJ. Equine Emergencies: Treatment and Procedures. 3rd ed. St. Louis, Missouri: Saunders; 2008. [Google Scholar]
- 68.Feige K, Schwarzwald CC, Bombeli TH. Comparison of unfractioned and low molecular weight heparin for prophylaxis of coagulopathies in 52 horses with colic: A randomised double-blind clinical trial. Equine Vet J. 2003;35:506–513. doi: 10.2746/042516403775600514. [DOI] [PubMed] [Google Scholar]
- 69.Hussni CA, Dornbusch PT, Alves ALG, et al. Avaliação clínica e angiográfica da vascularização consecutiva à ressecção experimental da jugular de equinos. Vet Zootec. 2006;13:163–168. [Google Scholar]
- 70.Sikri N, Bardia A. A history of streptokinase use in acute myocardial infarction. Tex Heart Inst J. 2007;34:318–327. [PMC free article] [PubMed] [Google Scholar]
- 71.Rang HP, Dale MM. Haemostasis and thrombosis. In: Rang HP, Dale MM, editors. Phamacology. New York, New York: Churchill Livingstone; 1987. pp. 280–296. [Google Scholar]
- 72.Dotter CT, Rosch J, Seaman AJ. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31–37. doi: 10.1148/111.1.31. [DOI] [PubMed] [Google Scholar]
- 73.Stegnar M, Kozak M, Sabovic M. D-dimer during intra-arterial low-dose streptokinase treatment of peripheral arterial occlusive disease. Fibrinolysis. 1994;8:105–107. (1994) [Google Scholar]
- 74.Baskurt OK, Farley RA, Meiselman HJ. Erythrocyte aggregation tendency and cellular properties in horse, human, and rat: A comparative study. Am J Physiol. 1997;273:H2604–H2612. doi: 10.1152/ajpheart.1997.273.6.H2604. [DOI] [PubMed] [Google Scholar]
- 75.Clark-Price SC, Cox JH, Bartoe JT, Davis EG. Use of dapsone in the treatment of Pneumocystis carinii pneumonia in a foal. J Am Vet Med Assoc. 2004;224:407–410. doi: 10.2460/javma.2004.224.407. [DOI] [PubMed] [Google Scholar]
- 76.Hilton H, Pusterla N. Intrapleural fibrinolytic therapy in the management of septic pleuropneumonia in a horse. Vet Rec. 2009;164:558–559. doi: 10.1136/vr.164.18.558. [DOI] [PubMed] [Google Scholar]