Table 3.
Synthesis of Polyfluoroaryl Oxiranes.
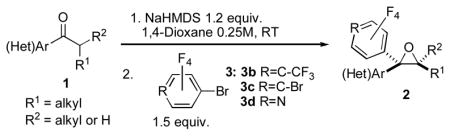
| ||||
|---|---|---|---|---|
| Entry | Ketone 1 | Bromide 3 | Product 2 | Yield, %[a] (dr) |
| 1 | 1a | 3b |
2n
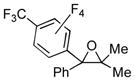
|
80 |
| 2 | 1a | 3c |
2o
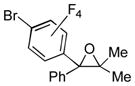
|
84 |
| 3 | 1a | 3d |
2p
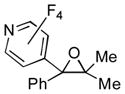
|
60 |
| 4 | 1l | 3b |
2q
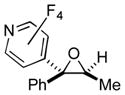
|
86 (85:15) |
| 5 | 1m | 3b |
2r
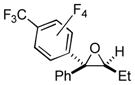
|
81 (77:23) |
| 6 | 1m | 3c |
2s

|
75 (90:10) |
| 7 | 1m | 3d |
2t
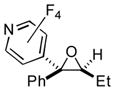
|
77 (90:10) |
Isolated yields of 0.5 mmol reactions.
