Abstract
The ability of differentiated thyroid cancer and adjacent thyroid cells to concentrate iodine is dependent on their expression of a functional NA+/I− symporter (NIS). Thyroid cancer is insensitive to 131I treatment if the thyroid cells lack the ability to concentrate iodide. Thus, in this study, we aimed to determine whether the NIS protein was differentially expressed in thyroid cancer and various surrounding tissues. We recruited 114 cases of papillary thyroid carcinoma (PTC) and divided them into two groups: 60 patients of 9 males and 51 females with a mean age of 49.55 years who had PTC with surrounding nodular goiter tissue (simplified as GNG), and 54 patients of 8 males and 46 females with a mean age of 45.78 years who had PTC with surrounding normal tissue (Gnormal) after total or near total thyroidectomy. Formalin-fixed and paraffin-embedded tissue sections were prepared for immunohistochemical staining of the NIS protein and semi-quantitative analysis. The NIS protein was expressed in the basolateral membrane of the normal epithelium, while PTC and nodular goiter cells expressed NIS in the cytoplasm and basolateral membrane. The expression levels of the NIS protein were higher in the adjacent normal tissues compared with those of the surrounding nodular goiter tissues (P=0.002) and expression levels of the NIS protein were higher in PTC tissues compared with the surrounding nodular goiter tissues (P=0.008). The data from this study indicate that cancer-surrounding tissues may play a significant role in mediating the sensitivity of PTC patients to radioactive iodine treatment.
Keywords: thyroid carcinoma, Na+I− symporter, nodular goiter, iodine, biomarker
Introduction
Thyroid cancer is the most common endocrine neoplasm. Histopathologically, thyroid cancers can be classified into papillary, follicular, medullary and anaplastic carcinomas. Papillary and follicular carcinomas are derived from thyroid follicular epithelial cells and account for the vast majority of thyroid cancer. These two types of thyroid cancers are differentiated tumors with low grades. The prognosis of thyroid cancer is associated with the histological type of the cancer and the stage at diagnosis. For papillary thyroid cancer (PTC), the overall prognosis is excellent following proper treatment, including thyroidectomy, lobectomy or radioactive iodine (RAI) therapy (1).
RAI ablation, which removes all remnant or residual normal thyroid tissues, is an important element of therapy following initial surgery in patients with papillary and follicular thyroid carcinomas (1). The underlying mechanism of RAI treatment of thyroid cancer is based on the ability of thyroid follicular cells to concentrate iodine, which is dependent on the functional Na+/I− symporter (NIS) (2). NIS is a transmembrane glycoprotein with a molecular weight of 87 kDa, which transports two Na+ for each I− into thyroid follicular cells. The loss of NIS expression in thyroid follicular cells may cause goiters or hypothyroidism. During thyroid cancer development, NIS expression has been reported to be reduced or lost. Thus, the detection of NIS expression was able to predict the outcome of RAI treatment in patients with thyroid cancer. Previous studies have demonstrated that the NIS protein was differentially expressed in differentiated thyroid carcinomas compared with normal thyroid tissues (2–4). By contrast, certain previous studies have found that PTC patients with normal NIS expression respond to RAI therapy (3,5,6). However, to date, few studies reporting NIS expression in PTC and their surrounding tissues have been published. Therefore, in the present study, we evaluated NIS expression in adjacent normal thyroid tissues in comparison to nodular goiter in PTC patients. The detection of NIS expression in surrounding normal thyroid tissues was able to predict the iodine uptake activity during RAI therapy of differentiated thyroid carcinomas.
Materials and methods
Study population
In this study, we first identified and reviewed the medical records from 600 patients who were diagnosed with PTC and underwent total or near-total thyroidectomy at the Affiliated Hospital of Qingdao Medical College between January 1, 2008 and January 1, 2011. Histology sections from these 600 patients were carefully re-examined by two pathologists for confirmation of the original diagnosis of PTC. Specimens of PTC and the surrounding normal tissues or the surrounding nodular goiter tissues were used in the current study, which generated two groups of cases: a group of 60 patients (52.63%; 9 males and 51 females with a mean age of 49.55±11.29 years) whose PTC had surrounding nodular goiter tissues (abbreviated as GNG) and a second group of 54 patients (47.37%; 8 males and 46 females with a mean age of 45.78±12.11 years) whose PTC had surrounding normal tissues (abbreviated as Gnormal) (Table I). 1. The study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao Medical College, Qingdao, China. Written informed patient consent was obtained from the patient.
Table I.
Expression of NIS protein and the clinicopathological characteristics of 114 PTC patients.
| IHS points in GNG cases
|
IHS points in Gnormal cases
|
|||||
|---|---|---|---|---|---|---|
| Characteristics | Total n=60 | Cancer tissue | Surrounding tissue | Total n=54 | Cancer tissue | Surrounding tissue |
| Total | 348 | 276 | 366 | 351 | ||
| Age (years) | ||||||
| ≥45 | 42 | 219 | 198 | 30 | 198 | 119 |
| <45 | 18 | 129 | 78 | 24 | 168 | 132 |
| Gender | ||||||
| Male | 9 | 48 | 33 | 8 | 30 | 33 |
| Female | 51 | 300 | 243 | 46 | 336 | 318 |
| Tumor size (cm) | ||||||
| ≥1 | 33 | 234 | 53 | 48 | 330 | 330 |
| <1 | 27 | 114 | 223 | 6 | 36 | 21 |
| N | ||||||
| + | 12 | 63 | 45 | 36 | 114 | 144 |
| - | 48 | 285 | 231 | 18 | 252 | 107 |
| DM | ||||||
| + | 0 | 0 | 0 | 3 | 24 | 36 |
| - | 60 | 348 | 276 | 51 | 342 | 315 |
| pTNM stage | ||||||
| I | 48 | 279 | 219 | 42 | 300 | 267 |
| II | 0 | 0 | 0 | 0 | 0 | 0 |
| III | 12 | 69 | 57 | 9 | 42 | 48 |
| IV | 0 | 0 | 0 | 3 | 24 | 36 |
PTC, papillary thyroid carcinoma; NIS, Na+/I− symporter; GNG, PTC with surrounding nodular goiter tissue; Gnormal, PTC with surrounding normal tissue; N, lymph node metastasis; DM, distant metastasis.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue blocks from PTCs with GNG and Gnormal were obtained from the Department of Pathology at the Affiliated Hospital of Qingdao Medical College, and prepared in 5-μm thick sections for immunohistochemistry. Briefly, all tissue sections were deparaffinized in xylene, rehydrated in graded alcohol (100-50%) and endogenous peroxidase activity was blocked in 3% H2O2 solution in methanol for 5 min and the sections were washed with PBS three times for 2 min each. Next, the sections were incubated with 20% normal serum for 30 min and then with a rabbit polyclonal anti-NIS antibody (#696557; American Basic Gene Associate Bioscience, Inc., Chicago, IL, USA) at a dilution of 1:400 in PBS at 4°C overnight. The next day, the sections were washed three times with PBS for 2 min each and incubated with a secondary antibody from a PV-6000 kit (Zhongshan Golden Bridge Biotechnology, Beijing, China) for 15 min at room temperature. Next, the sections were incubated with 3,3′-diaminobenzidine tetrahydrochloride solution (DAB; Zhongshan Golden Bridge Biotechnology) after washing three times with PBS. The color reaction was stopped after a suitable color had developed or after a maximum of 10 min. The sections were briefly counterstained with hematoxylin. Finally, all sections were washed with distilled water, dehydrated through ascending alcohol and xylene washes and mounted with cover slips with a drop of mounting medium. Both positive and negative controls were used for each sample of tumor tissues and surrounding tissues.
Review and score of the immunostained tissue sections
All the immunostained tissue sections were reviewed and scored under a microscope for expression and localization of NIS protein by two pathologists independently and blindly. The scores of each section were compared and if there was a discrepancy, the two pathologists reviewed them again and reached a consensus. Briefly, five high-powered fields under the microscope were randomly chosen and one hundred cells in each field were counted. The staining scores (IHS) were calculated by combining an estimate of the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score) (7). For the percentage of staining, 0 indicated no staining; 1, 1–10% of cells stained; 2, 11–50%; 3, 51–80%; 4, 81–100%. Staining intensity scores were as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The raw data were converted to the IHS by multiplying the quantity and staining intensity scores. The scores of IHS ranged from 0 to 12, with 0 indicating negative, 1 to 4 indicating weak, 5 to 8 indicating moderate and 9 to 12 indicating strong immunoreactivity. For multi-focal immunoreactivity or significant differences in staining intensities between foci, the average of the least and most intense staining was recorded.
Statistical analyses
All statistical analyses were performed with SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical independent sample t-tests or t’-tests were used to determine the correlation of NIS expression between tumor and normal tissues. P<0.05 was considered to indicate a statistically significant result.
Results
Clinicopathological characteristics
In this study, we collected a total of 114 cases of PTC and divided them into two groups: 60 patients with PTC whose resected tissues contained adjacent normal thyroid epithelium (Gnormal) and 54 patients with PTC whose surgical specimens contained nodular goiter tissues (GNG). We found that tumor size (P=0.0004) and lymph node metastases (P=0.0000) were significantly different between these two groups. Additionally, Gnormal patients had larger tumor sizes and more lymph node metastases than GNG patients (Tables I and II).
Table II.
Comparison of NIS expression with clinicopathological data between PTC, GNG and Gnormal tissues.
| Characteristic | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| Total | 0.008 | 0.675 | 0.075 | 0.002 | |
| Age (years) | |||||
| ≥45 | 0.394 | 0.483 | 0.800 | 0.003 | |
| <45 | 0.110 | 0.000 | 0.065 | 0.781 | 0.107 |
| Gender | |||||
| Male | 0.389 | 0.361 | 0.000 | 0.000 | |
| Female | 0.84 | 0.003 | 1.000 | 0.326 | 0.000 |
| Tumor size (cm) | |||||
| ≥1 | 0.000 | 1.000 | 0.693 | 0.002 | |
| <1 | 0.0004 | 0.879 | 0.037 | 0.310 | 0.358 |
| N | |||||
| + | 0.026 | 0.175 | 0.284 | 0.000 | |
| - | 0.000 | 0.037 | 0.107 | 0.111 | 0.162 |
| DM | |||||
| + | - | - | - | - | |
| - | 0.065 | 0.008 | 0.432 | 1.108 | 0.008 |
| pTNM stage | |||||
| I | 0.172 | 0.017 | 0.270 | 0.031 | 0.006 |
| II | - | - | - | - | |
| III | 0.278 | 0.725 | 0.417 | 0.696 | |
| IV | - | - | - | - |
NIS, Na+/I− symporter; PTC, papillary thyroid carcinoma; GNG, PTC with surrounding nodular goiter tissue; Gnormal, PTC with surrounding normal tissue; N, lymph node metastasis; DM, distant metastasis. P1, comparison of GNG and Gnormal groups; P2, comparison of NIS protein expression between cancer and GNG tissues; P3, comparison of NIS protein expression between cancer and Gnormal tissues; P4, comparison of NIS protein expression in cancer tissues between GNG and Gnormal groups; P5, comparison of NIS protein expression between and GNG and Gnormal tissues.
Expression of NIS
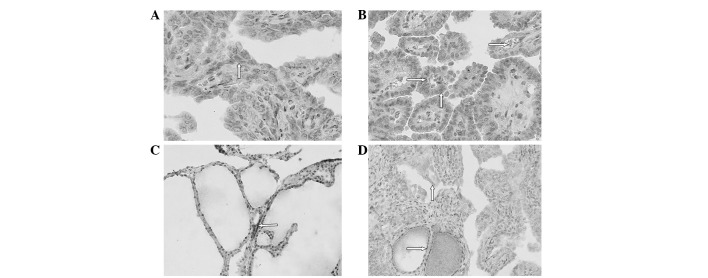
We then assessed NIS expression in these tissues and found that NIS protein was expressed in the baso-lateral membrane of the normal epithelium, while nodular goiter cells expressed NIS in the cytoplasm and occasionally in the basolateral membrane (Fig. 1). By contrast, NIS protein was mostly expressed in the cytoplasm and rarely in the basolateral membrane of PTC cells (Fig. 1). Based on the percentage of staining and the staining intensity of the NIS antibody, we summarized NIS expression levels as ‘points’ in each section of PTC, Gnormal and GNG tissues. The 114 cases of thyroid cancer exhibited NIS staining with a score ranging between 0 and 12 points. The total NIS protein scores of the PTC and GNG tissues were 348 and 276 points, respectively (P=0.008). In addition, we associated NIS expression with clinicopathological data from the PTC patients with surrounding GNG tissue. We found that age <45 years (P=0.000), female gender (P=0.003), tumor size ≥1 cm (P= 0.000), TNM stage I (P= 0.017) and lymph node and distant metastases (P=0.026 and P=0.008, respectively) were associated with NIS expression.
Figure 1.
Expression and localization of NIS protein in human thyroid tissues. (A) Immunohistochemical localization of NIS protein in the cytoplasm (⇧) (magnification, x200). (B) Immunohistochemical localization of NIS protein in the basolateral membrane (⇨) and cytoplasm (⇧) (magnification, x200). (C) NIS expression in the part basolateral membrane (⇦) in surrounding nodule goiter tissues (magnification, x200). (D) Immunohistochemical localization of NIS protein in the cytoplasm (ñ) in PTC tissue and surrounding normal thyroid tissue (⇨) (magnification, x200). NIS, Na+/I- symporter; PTC, papillary thyroid carcinoma.
IHS scores
Furthermore, as shown in Table II, the total scores of NIS expression in PTC and Gnormal tissues were 366 and 351 points, respectively, which was not statistically significant (P= 0.675). By contrast, the expression levels of NIS protein in Gnormal tissue of tumors <1 cm in size were lower (21/351 points) compared with cancerous tissue (36/366; P= 0.037). Moreover, NIS expression in cancerous tissue of Gnormal and GNG did not reach statistical significance (366 and 348 points, respectively, P=0.075), indicating that the cancer-surrounding tissues may play an important role in mediating the sensitivity of PTC patients to RAI treatment. Gnormal tissue from male patients had lower NIS expression (30/366 points) compared with GNG tissue (48/348 points; P=0.000). Indeed, the total score of NIS expression of GNG tissues were lower than that of Gnormal tissues (276 vs. 351 points; P= 0.002) (Table II) and were not dependent on gender (P=0.000), tumor size (P<0.05) or lymph node and distant metastasis (P<0.05).
Discussion
In the present study, we detected expression levels of NIS protein in cases of PTC with adjacent normal or adjacent nodular goiter tissues. We observed the differential expression of NIS protein among these three types of tissues, suggesting that adjacent thyroid tissue may play an important role in the uptake of iodide during RAI therapy and may aid the prediction of treatment outcomes. NIS is an integral plasma membrane glycoprotein that is mostly expressed in the thyroid gland and mediates the active transport of I− into the thyroid follicular cells, which is the crucial first step for thyroid hormone biosynthesis (8). Differentiated thyroid carcinoma (DTC) retains this iodide-concentrating ability due to the functional integrity of the NIS (2). However, certain DTCs are incapable of concentrating iodide and are therefore resistant to RAI therapy. The reason for this may be due to tumor or adjacent tissues that do not express functional NIS protein. Normally, NIS protein is localized in the cell membrane, otherwise, it loses the ability to uptake iodide (4,9).
The results of our current study demonstrated that the NIS protein was mostly localized to the cytoplasm of thyroid cancer, indicating non- or low-functional NIS protein, whereas adjacent normal glands or even goiters expressed NIS in the plasma membrane, suggesting a functional NIS protein. Localization of NIS protein at the basolateral plasma membrane is important for NIS function to transport iodide in the thyroid gland and radioiodide in thyroid cancer therapy. Dohán et al(9) demonstrated that improvements in 131I radioablation therapy for thyroid carcinoma patients resulted from the introduction of NIS targeting the plasma membrane. Another study showed that iodine supply also influenced expression and localization of human NIS (hNIS) protein (10). For example, thyroid nodules from the iodine-sufficient area had absent or only weak NIS protein expression, whereas almost all the nodules from the iodine-deficient area expressed NIS protein. Our current data agree with these previous data (9,10) and show that few cases of PTC cells expressed functional NIS protein in the bilateral membrane, indicating that the tumor cells had lost the ability to intake iodine. From this point of view, these tissues play an important role in predicting the sensitivity of RAI treatment.
Furthermore, we observed differential NIS expression in different surrounding tissues from PTC patients. This is the first study to report that expression levels of NIS protein were lower in surrounding nodular goiter tissues than in surrounding normal thyroid tissues. A previous study revealed that immunodetection of NIS protein predicted radioiodine uptake in thyroid cancer tissues (3), recurrent lesions (5), metastatic and recurrent disease (6). Thus, we suggest that the impaired NIS protein localization or various expression levels in the surrounding tissues may also affect the sensitivity of PTC to RAI therapy. Moreover, the current data further indicated that plasma membrane localization of NIS protein or induction of NIS cell membrane expression may improve the sensitivity of PTC to RAI therapy. Thus, detection of NIS protein expression and the localization of NIS in surrounding thyroid tissues may be useful to predict RAI therapy outcomes in PTC patients. In addition, based on NIS protein expression and localization, a physician administering nuclear medicine may be able to modify the 131I dose when treating PTC.
In conclusion, NIS protein was differentially expressed in surrounding normal thyroid tissues and nodular goiter tissues from PTC patients and may regulate the sensitivity of PTC patients to RAI therapy. Future studies should evaluate the association between NIS protein expression, sensitivity of RAI treatment and serum levels of iodine in PTC patients.
Acknowledgments
The authors would like to thank Professor Xiaoming Xing of the Department of Pathology, The Affiliated Hospital of Qingdao Medical College (Qingdao, China), for her excellent assistance with pathology.
References
- 1.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Arturi F, Russo D, Schlumberger M, du Villard JA, Caillou B, Vigneri P, Wicker R, Chiefari E, Suarez HG, Filetti S. Iodide symporter gene expression in human thyroid tumors. J Clin Endocrinol Metab. 1998;83:2493–2496. doi: 10.1210/jcem.83.7.4974. [DOI] [PubMed] [Google Scholar]
- 3.Caillou B, Troalen F, Baudin E, Talbot M, Filetti S, Schlumberger M, Bidart JM. Na+/I- symporter distribution in human thyroid tissues: an immunohistochemical study. J Clin Endocrinol Metab. 1998;83:4102–4106. doi: 10.1210/jcem.83.11.5262. [DOI] [PubMed] [Google Scholar]
- 4.Saito T, Endo T, Kawaguchi A, Ikeda M, Katoh R, Kawaoi A, Muramatsu A, Onaya T. Increased expression of the sodium/iodide symporter in papillary thyroid carcinomas. J Clin Invest. 1998;101:1296–1300. doi: 10.1172/JCI1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, Lee MC, Cho BY. Relationship between expression of the sodium/iodide symporter and I-131 uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001;28:639–645. doi: 10.1007/s002590100509. [DOI] [PubMed] [Google Scholar]
- 6.Castro MR, Bergert ER, Goellner JR, Hay ID, Morris JC. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. J Clin Endocrinol Metab. 2001;86:5627–5632. doi: 10.1210/jcem.86.11.8048. [DOI] [PubMed] [Google Scholar]
- 7.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 9.Dohán O, Baloch Z, Bánrévi Z, Livolsi V, Carrasco N. Rapid communication: predominant intracellular overexpression of the Na(+)/I(-) symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab. 2001;86:2697–2700. doi: 10.1210/jcem.86.6.7746. [DOI] [PubMed] [Google Scholar]
- 10.Scipioni A, Ferretti E, Soda G, Tosi E, Bruno R, Costante G, Meringolo D, Arturi F, Durante C, Amorosi A, Foschini MP, Nardi F, Russo D, Filetti S. hNIS protein in thyroid: the iodine supply influences its expression and localization. Thyroid. 2007;17:613–618. doi: 10.1089/thy.2007.0064. [DOI] [PubMed] [Google Scholar]